Organotypic Epithelial Raft Cultures as a Three-Dimensional In Vitro Model of Merkel Cell Carcinoma
- PMID: 35205840
- PMCID: PMC8870341
- DOI: 10.3390/cancers14041091
Organotypic Epithelial Raft Cultures as a Three-Dimensional In Vitro Model of Merkel Cell Carcinoma
Abstract
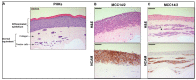
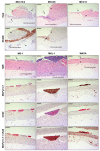
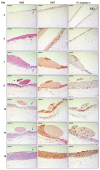
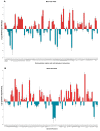
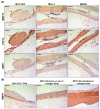
Merkel cell carcinoma (MCC) is a rare type of skin cancer for which an in vitro model is still lacking. MCC tumorigenesis is associated either with the integration of Merkel cell polyomavirus into the host genome, or with the accumulation of somatic mutations upon chronic exposure to UV light. Transgenic animals expressing the viral oncoproteins, which are constitutively expressed in virus-related MCC, do not fully recapitulate MCC. Although cell-line-derived xenografts have been established for the two subtypes of MCC, they still present certain limitations. Here, we generated organotypic epithelial raft cultures (OERCs) of MCC by using primary human keratinocytes and both virus-positive and virus-negative MCC cell lines. The primary human keratinocytes and the tumor cells were grown on top of a dermal equivalent. Histological and immunohistochemical examination of the rafts confirmed the growth of MCC cells. Furthermore, gene expression analysis revealed differences in the expression profiles of the distinct tumor cells and the keratinocytes at the transcriptional level. In summary, considering the limited availability of patient samples, OERCs of MCC may constitute a suitable model for evaluating the efficacy and selectivity of new drug candidates against MCC; moreover, they are a potential tool to study the oncogenic mechanisms of this malignancy.
Keywords: 3D cell culture model; Merkel cell carcinoma; Merkel cell polyomavirus; gene expression profile; non-melanoma skin cancer; raft culture.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
A Novel In Vitro Culture Model System to Study Merkel Cell Polyomavirus-Associated MCC Using Three-Dimensional Organotypic Raft Equivalents of Human Skin.Viruses. 2021 Jan 19;13(1):138. doi: 10.3390/v13010138. Viruses. 2021. PMID: 33478104 Free PMC article.
-
Merkel cell polyomavirus and non-Merkel cell carcinomas: guilty or circumstantial evidence?APMIS. 2020 Feb;128(2):104-120. doi: 10.1111/apm.13019. Epub 2020 Jan 28. APMIS. 2020. PMID: 31990105 Review.
-
Characterization of six Merkel cell polyomavirus-positive Merkel cell carcinoma cell lines: Integration pattern suggest that large T antigen truncating events occur before or during integration.Int J Cancer. 2019 Aug 15;145(4):1020-1032. doi: 10.1002/ijc.32280. Epub 2019 Apr 4. Int J Cancer. 2019. PMID: 30873613
-
Characterization of an early passage Merkel cell polyomavirus-positive Merkel cell carcinoma cell line, MS-1, and its growth in NOD scid gamma mice.J Virol Methods. 2013 Jan;187(1):6-14. doi: 10.1016/j.jviromet.2012.10.001. Epub 2012 Oct 18. J Virol Methods. 2013. PMID: 23085629 Free PMC article.
-
Histogenesis of Merkel Cell Carcinoma: A Comprehensive Review.Front Oncol. 2019 Jun 10;9:451. doi: 10.3389/fonc.2019.00451. eCollection 2019. Front Oncol. 2019. PMID: 31245285 Free PMC article. Review.
Cited by
-
Current In Vitro and In Vivo Models to Study MCPyV-Associated MCC.Viruses. 2022 Oct 7;14(10):2204. doi: 10.3390/v14102204. Viruses. 2022. PMID: 36298759 Free PMC article. Review.
-
Evaluation of Biological Activity of Natural Compounds: Current Trends and Methods.Molecules. 2022 Jul 13;27(14):4490. doi: 10.3390/molecules27144490. Molecules. 2022. PMID: 35889361 Free PMC article. Review.
References
-
- Harms P.W., Harms K.L., Moore P.S., DeCaprio J.A., Nghiem P., Wong M.K.K., Brownell I. International Workshop on Merkel Cell Carcinoma Research Working, G. The biology and treatment of Merkel cell carcinoma: Current understanding and research priorities. Nat. Rev. Clin. Oncol. 2018;15:763–776. doi: 10.1038/s41571-018-0103-2. - DOI - PMC - PubMed
-
- Paulson K.G., Park S.Y., Vandeven N.A., Lachance K., Thomas H., Chapuis A.G., Harms K.L., Thompson J.A., Bhatia S., Stang A., et al. Merkel cell carcinoma: Current US incidence and projected increases based on changing demographics. J. Am. Acad. Dermatol. 2018;78:457–463.e452. doi: 10.1016/j.jaad.2017.10.028. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

