Targeting Protein O-GlcNAcylation, a Link between Type 2 Diabetes Mellitus and Inflammatory Disease
- PMID: 35203353
- PMCID: PMC8870601
- DOI: 10.3390/cells11040705
Targeting Protein O-GlcNAcylation, a Link between Type 2 Diabetes Mellitus and Inflammatory Disease
Abstract
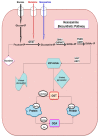
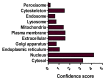
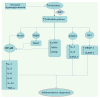
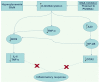
Unresolved hyperglycaemia, a hallmark of type 2 diabetes mellitus (T2DM), is a well characterised manifestation of altered fuel homeostasis and our understanding of its role in the pathologic activation of the inflammatory system continues to grow. Metabolic disorders like T2DM trigger changes in the regulation of key cellular processes such as cell trafficking and proliferation, and manifest as chronic inflammatory disorders with severe long-term consequences. Activation of inflammatory pathways has recently emerged as a critical link between T2DM and inflammation. A substantial body of evidence has suggested that this is due in part to increased flux through the hexosamine biosynthetic pathway (HBP). The HBP, a unique nutrient-sensing metabolic pathway, produces the activated amino sugar UDP-GlcNAc which is a critical substrate for protein O-GlcNAcylation, a dynamic, reversible post-translational glycosylation of serine and threonine residues in target proteins. Protein O-GlcNAcylation impacts a range of cellular processes, including inflammation, metabolism, trafficking, and cytoskeletal organisation. As increased HBP flux culminates in increased protein O-GlcNAcylation, we propose that targeting O-GlcNAcylation may be a viable therapeutic strategy for the prevention and management of glucose-dependent pathologies with inflammatory components.
Keywords: O-GlcNAcylation; diabetes; inflammation; metabolism.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Revascularisation of type 2 diabetics with coronary artery disease: Insights and therapeutic targeting of O-GlcNAcylation.Nutr Metab Cardiovasc Dis. 2021 May 6;31(5):1349-1356. doi: 10.1016/j.numecd.2021.01.017. Epub 2021 Feb 2. Nutr Metab Cardiovasc Dis. 2021. PMID: 33812732 Review.
-
Fueling the fire: emerging role of the hexosamine biosynthetic pathway in cancer.BMC Biol. 2019 Jul 4;17(1):52. doi: 10.1186/s12915-019-0671-3. BMC Biol. 2019. PMID: 31272438 Free PMC article. Review.
-
New insights: A role for O-GlcNAcylation in diabetic complications.Crit Rev Biochem Mol Biol. 2016 May-Jun;51(3):150-61. doi: 10.3109/10409238.2015.1135102. Epub 2016 Jan 24. Crit Rev Biochem Mol Biol. 2016. PMID: 26806492 Review.
-
Hexosamine biosynthetic pathway promotes the antiviral activity of SAMHD1 by enhancing O-GlcNAc transferase-mediated protein O-GlcNAcylation.Theranostics. 2021 Jan 1;11(2):805-823. doi: 10.7150/thno.50230. eCollection 2021. Theranostics. 2021. PMID: 33391506 Free PMC article.
-
The Hexosamine Biosynthesis Pathway: Regulation and Function.Genes (Basel). 2023 Apr 18;14(4):933. doi: 10.3390/genes14040933. Genes (Basel). 2023. PMID: 37107691 Free PMC article. Review.
Cited by
-
Role of Branched-Chain Amino Acid Metabolism in Type 2 Diabetes, Obesity, Cardiovascular Disease and Non-Alcoholic Fatty Liver Disease.Int J Mol Sci. 2022 Apr 13;23(8):4325. doi: 10.3390/ijms23084325. Int J Mol Sci. 2022. PMID: 35457142 Free PMC article. Review.
-
Emerging Role of Protein O-GlcNAcylation in Liver Metabolism: Implications for Diabetes and NAFLD.Int J Mol Sci. 2023 Jan 21;24(3):2142. doi: 10.3390/ijms24032142. Int J Mol Sci. 2023. PMID: 36768465 Free PMC article. Review.
-
O-GlcNAcylation: The Underestimated Emerging Regulators of Skeletal Muscle Physiology.Cells. 2022 May 30;11(11):1789. doi: 10.3390/cells11111789. Cells. 2022. PMID: 35681484 Free PMC article. Review.
-
Characterizing Adrenergic Regulation of Glucose Transporter 4-Mediated Glucose Uptake and Metabolism in the Heart.JACC Basic Transl Sci. 2023 Feb 22;8(6):638-655. doi: 10.1016/j.jacbts.2022.11.008. eCollection 2023 Jun. JACC Basic Transl Sci. 2023. PMID: 37426525 Free PMC article.
-
Purine Metabolism and Hexosamine Biosynthetic Pathway Abnormalities in Diarrheal Weaned Piglets Identified Using Metabolomics.Animals (Basel). 2024 Feb 5;14(3):522. doi: 10.3390/ani14030522. Animals (Basel). 2024. PMID: 38338165 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

