Efficacy and Safety of Finerenone in Chronic Kidney Disease: A Systematic Review and Meta-Analysis of Randomized Clinical Trials
- PMID: 35197856
- PMCID: PMC8859447
- DOI: 10.3389/fphar.2022.819327
Efficacy and Safety of Finerenone in Chronic Kidney Disease: A Systematic Review and Meta-Analysis of Randomized Clinical Trials
Abstract
Background: Chronic kidney disease (CKD) is a global public health issue. In recent years, the effectiveness of finerenone for treatment of CKD has been the subject of considerable debate. The main objective of the current meta-analysis was to validate the clinical efficacy and safety of finerenone in patients with CKD. Methods: Seven databases were searched for randomized controlled trials (RCTs) comparing finerenone with placebo in patients with CKD. Data from eligible studies were extracted, and the Cochrane risk of bias tool utilized for evaluating the methodological quality of RCTs. The effect size was estimated using the risk ratio (RR) and mean difference (MD) with 95% confidence interval (CI). Results: Five trials (n = 13,078) were included. Compared to placebo groups, the urinary albumin-to-creatinine ratio (UACR) mean from the baseline was significantly lower [MD -0.30 (95% CI -0.32, -0.28), p < 0.00001], while a decrease in the estimated glomerular filtration rate (eGFR) from baseline was significantly higher [MD -2.44 (95% CI -2.82, -2.05), p < 0.00001] for the finerenone groups. Furthermore, the proportion of patients with decreased eGFR (≥40%) post-baseline was significantly lower [RR 0.85 (95% CI 0.78, 0.93), p = 0.0002], along with end-stage kidney disease (ESKD) [RR 0.80 (95% CI 0.65, 0.99), p = 0.04] and cardiovascular events (CVs) [RR 0.88 (95% CI 0.80, 0.95), p < 0.003] in the finerenone groups. In terms of safety, the increase in the serum potassium concentration and incidence of hyperkalemia was significantly higher for the finerenone groups [MD 0.17 (95% CI 0.10, 0.24), p < 0.00001; RR 2.03 (95% CI 1.83, 2.26), p < 0.00001, respectively], but the incidence of adverse events (AEs) was similar to placebo [RR 1.00 (95% CI 0.98-1.01), p = 0.67]. In all cases, the results were rated as providing moderate-quality or high-quality evidence. Conclusion: Data from our meta-analysis suggest that finerenone confers significant renal and cardiovascular benefits in patients with CKD. While higher risk of hyperkalemia was observed with finerenone than placebo, differences in AEs were not significant. Finerenone may therefore present a novel promising therapeutic agent for patients with CKD. Systematic Review Registration: [https://inplasy.com/inplasy-2021-9-0020/], identifier [INPLASY202190020].
Keywords: chronic kidney disease; finerenone; meta-analysis; randomized clinical trials; systematic review.
Copyright © 2022 Zhang, Bao, Zheng, Wang and Sun.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

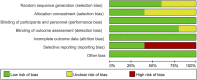








Similar articles
-
Efficacy and safety of finerenone in chronic kidney disease associated with type 2 diabetes: a systematic review and meta-analysis of randomized clinical trials.Eur J Clin Pharmacol. 2022 Dec;78(12):1877-1887. doi: 10.1007/s00228-022-03408-w. Epub 2022 Oct 22. Eur J Clin Pharmacol. 2022. PMID: 36273065 Review.
-
Efficacy and safety of finerenone in patients with chronic kidney disease: a systematic review with meta-analysis and trial sequential analysis.Ann Palliat Med. 2021 Jul;10(7):7428-7439. doi: 10.21037/apm-21-763. Ann Palliat Med. 2021. PMID: 34353035
-
Meta-Analysis of the Efficacy and Safety of Finerenone in Diabetic Kidney Disease.Kidney Blood Press Res. 2022;47(4):219-228. doi: 10.1159/000521908. Epub 2022 Jan 14. Kidney Blood Press Res. 2022. PMID: 35034019
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
A Systematic Review and Meta-Analysis on the Efficacy and Safety of Finerenone Therapy in Patients with Cardiovascular and Chronic Kidney Diseases in Type 2 Diabetes Mellitus.Cureus. 2023 Jul 11;15(7):e41746. doi: 10.7759/cureus.41746. eCollection 2023 Jul. Cureus. 2023. PMID: 37575756 Free PMC article. Review.
Cited by
-
Finerenone in type 2 diabetes and renal outcomes: A random-effects model meta-analysis.Front Endocrinol (Lausanne). 2023 Jan 20;14:1114894. doi: 10.3389/fendo.2023.1114894. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 36742404 Free PMC article.
-
Effect of Avenciguat on Albuminuria in Patients with CKD: Two Randomized Placebo-Controlled Trials.J Am Soc Nephrol. 2024 May 25;35(9):1227-39. doi: 10.1681/ASN.0000000000000418. Online ahead of print. J Am Soc Nephrol. 2024. PMID: 38795055
-
Primary Aldosteronism and the Role of Mineralocorticoid Receptor Antagonists for the Heart and Kidneys.Annu Rev Med. 2023 Jan 27;74:217-230. doi: 10.1146/annurev-med-042921-100438. Epub 2022 Nov 14. Annu Rev Med. 2023. PMID: 36375469 Free PMC article. Review.
-
Low-Protein Diets, Malnutrition, and Bone Metabolism in Chronic Kidney Disease.Nutrients. 2024 Sep 13;16(18):3098. doi: 10.3390/nu16183098. Nutrients. 2024. PMID: 39339698 Free PMC article. Review.
-
Effects of Finerenone, a Novel Nonsteroidal Mineralocorticoid Receptor Antagonist, on Cardiovascular Disease, Chronic Kidney Disease, and Blood Pressure.Curr Cardiol Rep. 2022 Oct;24(10):1251-1259. doi: 10.1007/s11886-022-01750-0. Epub 2022 Aug 4. Curr Cardiol Rep. 2022. PMID: 35925515 Review.
References
-
- Agarwal R., Kolkhof P., Bakris G., Bauersachs J., Haller H., Wada T., et al. (2021c). Steroidal and Non-steroidal Mineralocorticoid Receptor Antagonists in Cardiorenal Medicine. Eur. Heart J. 42 (2), 152–161. 10.1093/eurheartj/ehaa736 10.1093/eurheartj/ehaa736 | Google Scholar - DOI - DOI - PMC - PubMed
-
- Agarwal R., Filippatos G., Pitt B., Anker S. D., Rossing P., Joseph A., et al. (2021a). Cardiovascular and Kidney Outcomes with Finerenone in Patients with Type 2 Diabetes and Chronic Kidney Disease: the FIDELITY Pooled Analysis. Eur. Heart J. Epub ahead of print, ehab777. 10.1093/eurheartj/ehab777 PubMed Abstract | 10.1093/eurheartj/ehab777 | Google Scholar - DOI - DOI - PMC - PubMed
-
- Agarwal R., Joseph A., Anker S. D., Filippatos G., Rossing P., Ruilope L. M., et al. (2021b). Hyperkalemia Risk with Finerenone: Results from the FIDELIO-DKD Trial. Jasn 33, 225–237. 10.1681/asn.2021070942 PubMed Abstract | 10.1681/asn.2021070942 | Google Scholar - DOI - DOI - PMC - PubMed
-
- Bakris G. L., Agarwal R., Chan J. C., Cooper M. E., Gansevoort R. T., Haller H., et al. (2015). Effect of Finerenone on Albuminuria in Patients with Diabetic Nephropathy: A Randomized Clinical Trial. Jama 314 (9), 884–894. 10.1001/jama.2015.10081 PubMed Abstract | 10.1001/jama.2015.10081 | Google Scholar - DOI - DOI - PubMed
-
- Bakris G. L., Agarwal R., Anker S. D., Pitt B., Ruilope L. M., Rossing P., et al. (2020). Effect of Finerenone on Chronic Kidney Disease Outcomes in Type 2 Diabetes. N. Engl. J. Med. 383 (23), 2219–2229. 10.1056/NEJMoa2025845 PubMed Abstract | 10.1056/NEJMoa2025845 | Google Scholar - DOI - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

