Animal studies for the evaluation of in situ tissue-engineered vascular grafts - a systematic review, evidence map, and meta-analysis
- PMID: 35197483
- PMCID: PMC8866508
- DOI: 10.1038/s41536-022-00211-0
Animal studies for the evaluation of in situ tissue-engineered vascular grafts - a systematic review, evidence map, and meta-analysis
Abstract
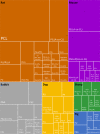
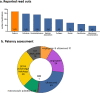
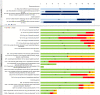
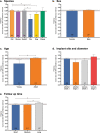
Vascular in situ tissue engineering (TE) is an approach that uses bioresorbable grafts to induce endogenous regeneration of damaged blood vessels. The evaluation of newly developed in situ TE vascular grafts heavily relies on animal experiments. However, no standard for in vivo models or study design has been defined, hampering inter-study comparisons and translational efficiency. To provide input for formulating such standard, the goal of this study was to map all animal experiments for vascular in situ TE using off-the-shelf available, resorbable synthetic vascular grafts. A literature search (PubMed, Embase) yielded 15,896 studies, of which 182 studies met the inclusion criteria (n = 5,101 animals). The reports displayed a wide variety of study designs, animal models, and biomaterials. Meta-analysis on graft patency with subgroup analysis for species, age, sex, implantation site, and follow-up time demonstrated model-specific variations. This study identifies possibilities for improved design and reporting of animal experiments to increase translational value.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Inflammatory and regenerative processes in bioresorbable synthetic pulmonary valves up to two years in sheep-Spatiotemporal insights augmented by Raman microspectroscopy.Acta Biomater. 2021 Nov;135:243-259. doi: 10.1016/j.actbio.2021.09.005. Epub 2021 Sep 10. Acta Biomater. 2021. PMID: 34509697
-
Considerations in the Development of Small-Diameter Vascular Graft as an Alternative for Bypass and Reconstructive Surgeries: A Review.Cardiovasc Eng Technol. 2020 Oct;11(5):495-521. doi: 10.1007/s13239-020-00482-y. Epub 2020 Aug 18. Cardiovasc Eng Technol. 2020. PMID: 32812139 Review.
-
Engineered Prevascularization for Oral Tissue Grafting: A Systematic Review.Tissue Eng Part B Rev. 2020 Aug;26(4):383-398. doi: 10.1089/ten.TEB.2020.0093. Epub 2020 Aug 7. Tissue Eng Part B Rev. 2020. PMID: 32597330
-
In situ tissue regeneration using a novel tissue-engineered, small-caliber vascular graft without cell seeding.J Thorac Cardiovasc Surg. 2008 Oct;136(4):900-7. doi: 10.1016/j.jtcvs.2008.02.058. Epub 2008 Jul 3. J Thorac Cardiovasc Surg. 2008. PMID: 18954628
-
Review of Vascular Graft Studies in Large Animal Models.Tissue Eng Part B Rev. 2018 Apr;24(2):133-143. doi: 10.1089/ten.TEB.2017.0350. Epub 2017 Nov 30. Tissue Eng Part B Rev. 2018. PMID: 28978267 Free PMC article. Review.
Cited by
-
Multiscale structure and function of the aortic valve apparatus.Physiol Rev. 2024 Oct 1;104(4):1487-1532. doi: 10.1152/physrev.00038.2022. Epub 2023 Sep 21. Physiol Rev. 2024. PMID: 37732828 Free PMC article. Review.
-
Controlled and Synchronised Vascular Regeneration upon the Implantation of Iloprost- and Cationic Amphiphilic Drugs-Conjugated Tissue-Engineered Vascular Grafts into the Ovine Carotid Artery: A Proteomics-Empowered Study.Polymers (Basel). 2022 Nov 26;14(23):5149. doi: 10.3390/polym14235149. Polymers (Basel). 2022. PMID: 36501545 Free PMC article.
-
Manufacturing and validation of small-diameter vascular grafts: A mini review.iScience. 2024 Apr 29;27(6):109845. doi: 10.1016/j.isci.2024.109845. eCollection 2024 Jun 21. iScience. 2024. PMID: 38799581 Free PMC article. Review.
-
Valvulogenesis of a living, innervated pulmonary root induced by an acellular scaffold.Commun Biol. 2023 Oct 7;6(1):1017. doi: 10.1038/s42003-023-05383-z. Commun Biol. 2023. PMID: 37805576 Free PMC article.
-
The effect of chronic kidney disease on tissue formation of in situ tissue-engineered vascular grafts.APL Bioeng. 2023 May 23;7(2):026107. doi: 10.1063/5.0138808. eCollection 2023 Jun. APL Bioeng. 2023. PMID: 37234843 Free PMC article.
References
-
- Joseph P, et al. Reducing the global burden of cardiovascular disease, part 1: The epidemiology and risk factors. Circ. Res. 2017;121:677–694. - PubMed
-
- Chlupác J, Filová E, Bacáková L. Blood vessel replacement: 50 years of development and tissue engineering paradigms in vascular surgery. Physiol. Res. 2009;58(Suppl. 2):S119–S139. - PubMed
-
- Norgren L, et al. Inter-society consensus for the management of peripheral arterial disease. Int. Angiol. 2007;26:81–157. - PubMed
Publication types
Grants and funding
- 114024119/ZonMw (Netherlands Organisation for Health Research and Development)
- 114024119/ZonMw (Netherlands Organisation for Health Research and Development)
- 436001003/ZonMw (Netherlands Organisation for Health Research and Development)
- 114024119/ZonMw (Netherlands Organisation for Health Research and Development)
- 024.003.013/Nederlandse Organisatie voor Wetenschappelijk Onderzoek (Netherlands Organisation for Scientific Research)
LinkOut - more resources
Full Text Sources
Miscellaneous

