Comparison between methylprednisolone infusion and dexamethasone in COVID-19 ARDS mechanically ventilated patients
- PMID: 35194371
- PMCID: PMC8853130
- DOI: 10.1186/s43162-022-00113-z
Comparison between methylprednisolone infusion and dexamethasone in COVID-19 ARDS mechanically ventilated patients
Abstract
Background: Coronavirus disease 2019 (COVID-19) causing severe acute respiratory distress syndrome caused by coronavirus 2 (SARS-CoV-2) still has no solid effective therapy.From previous studies, dexamethasone has led to a decrease in mortality in patients who required oxygen supplementation mainly invasive mechanical ventilation; at the same time, it is unknown if another corticosteroid can be effective when used and what is the optimal dose and its duration, to achieve improvement in clinical outcome.The cornerstone of the study was to compare the differences in clinical outcome and laboratory results in intensive care patients with SARS-CoV-2 pneumonia treated with dexamethasone 6 mg/day: doses versus those treated with methylprednisolone 2 mg/kg/day infusion.
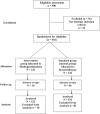
Materials and methods: A prospective cohort study with a survival analysis of 414 patients diagnosed with severe COVID-19 pneumonia confirmed by polymerase chain reaction, for SARS-CoV-2 according to the Berlin definition of ARDS, who were admitted in the intensive care unit in the Helwan University Hospitals; the duration is from June 2020 till October 2021.Patients included in the study were mechanically ventilated with radiological confirmation of pneumonia by chest tomography; patients were included in the study according to the Berlin definition of ARDS and met the inclusion criteria of the study; 222 patients were treated with methylprednisolone infusion with a dose of 2 mg/kg/day versus 192 patients treated with dexamethasone 6 mg/day; both groups were treated for 10 days and were mechanically ventilated; the clinical out come and differences in the laboratory results were evaluated during the 10-day course for each group.
Results: Four hundred fourteen patients had COVID-19 pneumonia, diagnosed and confirmed by ground glass opacities in chest tomography and arterial partial pressure of oxygen/inspired oxygen and fraction of inspired oxygen (P/F ratio) less than 300.Two hundred twenty-two patients received methylprednisolone infusion at a dose of 2 mg/kg/day, and 192 patients received dexamethasone 6 mg daily; both groups were treated for 10 days.Inflammatory markers for cytokine storm were improved in the methylprednisolone group in comparison to the patients who were given dexamethasone when comparing the on-admission markers to the results of the inflammatory markers after 10 days, like ferritin after 10 days in methylprednisolone group 292.26 ± 330.10 versus the dexa group 648.10 ± 329.09 (p value < 0.001).D-dimer in the methylprednisolone group was 1301.75 ± 1515.51 versus 2523.78 ± 843.18 in the dexa group (p value < 0.001); CRP was 49.65 ± 19.91 in the methylprednisolone group versus 100.54 ± 36.75 (p value < 0.001) in the dexa group; LDH after 10 days in methylprednisolone group was 345.09 ± 128.31, and in the dexa group, it was 731.87 ± 195.09 (p value < 0.001); neutrophil to lymphocyte ratio (N:L ratio) after 10 days of treatment in the methylprednisolone group was 17.27 ± 5.09 versus 26.68 ± 7.19 (p value < 0.001) in the dexa group; also, the length of stay was shorter in the methylprednisolone group (7.33 ± 1.71) versus in the dexa group (19.43 ± 5.42) (p value < 0.001), together with mechanical ventilation MV days which are 3.82 ± 1.14 in the methyl group versus 16.57 ± 4.71 in the dexa group (p value < 0.001).Also, the radiological findings are improved in the methyl group (20.3%) versus the dexa group (73.4%) with p value < 0.001, and discharge from ICU in the methyl group was 79.7% versus 26.6% in the dexa group with p value < 0.001.
Conclusions: Treatment of severe COVID-19 pneumonia, Patients who were mechanically ventilated with methylprednisolone infusion 2 mg/kg/day for 10 days versus dexamethasone 6 mg for 10 days showed a statistically significant improvement in the MV days and length of stay in the intensive care unit, together with the overall mortality and severity inflammatory markers of cytokine storm c-reactive protein (CRP), D-dimer, ferritin, LDH, and N:L ratio.
Keywords: ARDS; COVID-19 pneumonia; Mechanical ventilation; Steroids.
© The Author(s) 2022.
Conflict of interest statement
Competing interestsThe authors declare that they have no competing interests.
Figures
Similar articles
-
Dexamethasone vs methylprednisolone high dose for Covid-19 pneumonia.PLoS One. 2021 May 25;16(5):e0252057. doi: 10.1371/journal.pone.0252057. eCollection 2021. PLoS One. 2021. PMID: 34033648 Free PMC article. Clinical Trial.
-
Multi-centre, three arm, randomized controlled trial on the use of methylprednisolone and unfractionated heparin in critically ill ventilated patients with pneumonia from SARS-CoV-2 infection: A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Aug 17;21(1):724. doi: 10.1186/s13063-020-04645-z. Trials. 2020. PMID: 32807241 Free PMC article.
-
A Phase I/II Clinical Trial to evaluate the efficacy of baricitinib to prevent respiratory insufficiency progression in onco-hematological patients affected with COVID19: A structured summary of a study protocol for a randomised controlled trial.Trials. 2021 Feb 5;22(1):116. doi: 10.1186/s13063-021-05072-4. Trials. 2021. PMID: 33546739 Free PMC article.
-
Effect of dexamethasone in patients with ARDS and COVID-19 - prospective, multi-centre, open-label, parallel-group, randomised controlled trial (REMED trial): A structured summary of a study protocol for a randomised controlled trial.Trials. 2021 Mar 1;22(1):172. doi: 10.1186/s13063-021-05116-9. Trials. 2021. PMID: 33648568 Free PMC article.
-
Effect of methylprednisolone in reducing severe COVID-19 and mortality in high-risk patients: A retrospective study.SAGE Open Med. 2024 Sep 9;12:20503121241276683. doi: 10.1177/20503121241276683. eCollection 2024. SAGE Open Med. 2024. PMID: 39257516 Free PMC article. Review.
Cited by
-
Innate Immunity in Protection and Pathogenesis During Coronavirus Infections and COVID-19.Annu Rev Immunol. 2024 Jun;42(1):615-645. doi: 10.1146/annurev-immunol-083122-043545. Annu Rev Immunol. 2024. PMID: 38941608 Review.
-
Host-Based Treatments for Severe COVID-19.Curr Issues Mol Biol. 2023 Apr 5;45(4):3102-3121. doi: 10.3390/cimb45040203. Curr Issues Mol Biol. 2023. PMID: 37185727 Free PMC article. Review.
-
Prolonged higher dose methylprednisolone versus conventional dexamethasone in COVID-19 pneumonia: a randomised controlled trial (MEDEAS).Eur Respir J. 2023 Apr 20;61(4):2201514. doi: 10.1183/13993003.01514-2022. Print 2023 Apr. Eur Respir J. 2023. PMID: 36356972 Free PMC article. Clinical Trial.
-
A systematic review and meta-analysis of glucocorticoids treatment in severe COVID-19: methylprednisolone versus dexamethasone.BMC Infect Dis. 2023 May 5;23(1):290. doi: 10.1186/s12879-023-08280-2. BMC Infect Dis. 2023. PMID: 37147596 Free PMC article.
-
Clinical Characteristics and Outcome of Hospitalized COVID-19 Patients Treated with Standard Dose of Dexamethasone or High Dose of Methylprednisolone.Biomedicines. 2022 Jun 29;10(7):1548. doi: 10.3390/biomedicines10071548. Biomedicines. 2022. PMID: 35884852 Free PMC article.
References
-
- World Health Organization (2020). WHO announces COVID-19 outbreak a pandemic. Available from: https://www.euro.who.int/en/health-topics/health-emergencies/coronavirus.... Accessed 7 Dec 2020
-
- World Health Organization (2020). Statement on the second meeting of the International Health Regulations (2005) Emergency Committee regarding the outbreak of novel coronavirus (2019-nCoV). Available from: https://who.int/news/item/30–01–2020statement-on-the-second-meeting-of-t.... Accessed 11 Dec 2020
-
- World Health Organization (2019). Clinical management of severe acute respiratory infection when Middle East respiratory syndrome coronavirus (MERS-COV) infection is suspected; interim guidance. Available from: https://apps.who.int/iris/handle/10665/178529. Accessed 3 Jan 2021
-
- Worldmeter, COVID-19 Coronavirus Pandemic. 2020. J Availabe from: https://www.worldometers.Info/. Accessed 15 Jan 2021
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous