Early programming of reproductive health and fertility: novel neuroendocrine mechanisms and implications in reproductive medicine
- PMID: 35187579
- PMCID: PMC9071071
- DOI: 10.1093/humupd/dmac005
Early programming of reproductive health and fertility: novel neuroendocrine mechanisms and implications in reproductive medicine
Abstract
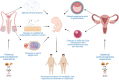
Background: According to the Developmental Origins of Health and Disease (DOHaD) hypothesis, environmental changes taking place during early maturational periods may alter normal development and predispose to the occurrence of diverse pathologies later in life. Indeed, adverse conditions during these critical developmental windows of high plasticity have been reported to alter the offspring developmental trajectory, causing permanent functional and structural perturbations that in the long term may enhance disease susceptibility. However, while solid evidence has documented that fluctuations in environmental factors, ranging from nutrient availability to chemicals, in early developmental stages (including the peri-conceptional period) have discernible programming effects that increase vulnerability to develop metabolic perturbations, the impact and eventual mechanisms involved, of such developmental alterations on the reproductive phenotype of offspring have received less attention.
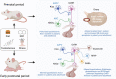
Objective and rationale: This review will summarize recent advances in basic and clinical research that support the concept of DOHaD in the context of the impact of nutritional and hormonal perturbations, occurring during the periconceptional, fetal and early postnatal stages, on different aspects of reproductive function in both sexes. Special emphasis will be given to the effects of early nutritional stress on the timing of puberty and adult gonadotropic function, and to address the underlying neuroendocrine pathways, with particular attention to involvement of the Kiss1 system in these reproductive perturbations. The implications of such phenomena in terms of reproductive medicine will also be considered.
Search methods: A comprehensive MEDLINE search, using PubMed as main interface, of research articles and reviews, published mainly between 2006 and 2021, has been carried out. Search was implemented using multiple terms, focusing on clinical and preclinical data from DOHaD studies, addressing periconceptional, gestational and perinatal programming of reproduction. Selected studies addressing early programming of metabolic function have also been considered, when relevant.
Outcomes: A solid body of evidence, from clinical and preclinical studies, has documented the impact of nutritional and hormonal fluctuations during the periconceptional, prenatal and early postnatal periods on pubertal maturation, as well as adult gonadotropic function and fertility. Furthermore, exposure to environmental chemicals, such as bisphenol A, and maternal stress has been shown to negatively influence pubertal development and gonadotropic function in adulthood. The underlying neuroendocrine pathways and mechanisms involved have been also addressed, mainly by preclinical studies, which have identified an, as yet incomplete, array of molecular and neurohormonal effectors. These include, prominently, epigenetic regulatory mechanisms and the hypothalamic Kiss1 system, which likely contribute to the generation of reproductive alterations in conditions of early nutritional and/or metabolic stress. In addition to the Kiss1 system, other major hypothalamic regulators of GnRH neurosecretion, such as γ-aminobutyric acid and glutamate, may be targets of developmental programming.
Wider implications: This review addresses an underdeveloped area of reproductive biology and medicine that may help to improve our understanding of human reproductive disorders and stresses the importance, and eventual pathogenic impact, of early determinants of puberty, adult reproductive function and fertility.
Keywords: early programming; fertility; gestational stage; kisspeptins; neuroendocrine mechanisms; nutrition; peri-conceptional stage; perinatal stage; puberty.
© The Author(s) 2022. Published by Oxford University Press on behalf of European Society of Human Reproduction and Embryology.
Figures
Similar articles
-
Disentangling puberty: novel neuroendocrine pathways and mechanisms for the control of mammalian puberty.Hum Reprod Update. 2017 Nov 1;23(6):737-763. doi: 10.1093/humupd/dmx025. Hum Reprod Update. 2017. PMID: 28961976 Review.
-
Nutritional control of puberty in the bovine female: prenatal and early postnatal regulation of the neuroendocrine system.Domest Anim Endocrinol. 2020 Oct;73:106434. doi: 10.1016/j.domaniend.2020.106434. Epub 2020 Jan 10. Domest Anim Endocrinol. 2020. PMID: 32115309 Review.
-
Environmentally Relevant Perinatal Exposures to Bisphenol A Disrupt Postnatal Kiss1/NKB Neuronal Maturation and Puberty Onset in Female Mice.Environ Health Perspect. 2019 Oct;127(10):107011. doi: 10.1289/EHP5570. Epub 2019 Oct 25. Environ Health Perspect. 2019. PMID: 31652106 Free PMC article.
-
Reproduction Symposium: developmental programming of reproductive and metabolic health.J Anim Sci. 2014 Aug;92(8):3199-210. doi: 10.2527/jas.2014-7637. J Anim Sci. 2014. PMID: 25074449 Free PMC article. Review.
-
Novel mechanisms for the metabolic control of puberty: implications for pubertal alterations in early-onset obesity and malnutrition.J Endocrinol. 2019 Aug;242(2):R51-R65. doi: 10.1530/JOE-19-0223. J Endocrinol. 2019. PMID: 31189134 Review.
Cited by
-
Effects of prenatal stress on reproductive function of male offspring through the KISS1 system.Endocr Connect. 2024 Sep 13;13(10):e240027. doi: 10.1530/EC-24-0027. Print 2024 Oct 1. Endocr Connect. 2024. PMID: 39140811 Free PMC article.
-
Maternal intake of folate and folic acid during pregnancy and markers of male fecundity: A population-based cohort study.Andrology. 2023 Mar;11(3):537-550. doi: 10.1111/andr.13364. Epub 2022 Dec 26. Andrology. 2023. PMID: 36524586 Free PMC article.
-
Disruptions in Hypothalamic-Pituitary-Gonadal Axis Development and Their IgG Modulation after Prenatal Systemic Inflammation in Male Rats.Int J Mol Sci. 2023 Feb 1;24(3):2726. doi: 10.3390/ijms24032726. Int J Mol Sci. 2023. PMID: 36769048 Free PMC article.
-
Cardiovascular health of offspring conceived by assisted reproduction technology: a comprehensive review.Front Cardiovasc Med. 2024 Jan 16;11:1287060. doi: 10.3389/fcvm.2024.1287060. eCollection 2024. Front Cardiovasc Med. 2024. PMID: 38292241 Free PMC article. Review.
-
The Role of Kisspeptin in the Control of the Hypothalamic-Pituitary-Gonadal Axis and Reproduction.Front Endocrinol (Lausanne). 2022 Jun 28;13:925206. doi: 10.3389/fendo.2022.925206. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35837314 Free PMC article. Review.
References
-
- Abbott DH, Dumesic DA, Eisner JR, Colman RJ, Kemnitz JW.. Insights into the development of polycystic ovary syndrome (PCOS) from studies of prenatally androgenized female rhesus monkeys. Trends Endocrinol Metab 1998;9:62–67. - PubMed
-
- Abecia JA, Casao A, Pascual-Alonso M, Lobon S, Aguayo-Ulloa LA, Meikle A, Forcada F, Sosa C, Marin RH, Silva MA. et al. The effect of periconceptional undernutrition of sheep on the cognitive/emotional response and oocyte quality of offspring at 30 days of age. J Dev Orig Health Dis 2014;5:79–87. - PubMed

