Dissecting in Vitro the Activation of Human Immune Response Induced by Shigella sonnei GMMA
- PMID: 35186786
- PMCID: PMC8851470
- DOI: 10.3389/fcimb.2022.767153
Dissecting in Vitro the Activation of Human Immune Response Induced by Shigella sonnei GMMA
Abstract
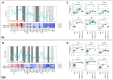
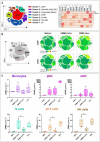
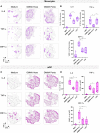
Generalized Modules for Membrane Antigens (GMMA) are outer membrane exosomes purified from Gram-negative bacteria genetically mutated to increase blebbing and reduce risk of reactogenicity. This is commonly achieved through modification of the lipid A portion of lipopolysaccharide. GMMA faithfully resemble the bacterial outer membrane surface, and therefore represent a powerful and flexible platform for vaccine development. Although GMMA-based vaccines have been demonstrated to induce a strong and functional antibody response in animals and humans maintaining an acceptable reactogenicity profile, the overall impact on immune cells and their mode of action are still poorly understood. To characterize the GMMA-induced immune response, we stimulated human peripheral blood mononuclear cells (hPBMCs) with GMMA from Shigella sonnei. We studied GMMA both with wild-type hexa-acylated lipid A and with the corresponding less reactogenic penta-acylated form. Using multicolor flow cytometry, we assessed the activation of immune cell subsets and we profiled intracellular cytokine production after GMMA stimulation. Moreover, we measured the secretion of thirty cytokines/chemokines in the cell culture supernatants. Our data indicated activation of monocytes, dendritic, NK, B, and γδ T cells. Comparison of the cytokine responses showed that, although the two GMMA have qualitatively similar profiles, GMMA with modified penta-acylated lipid A induced a lower production of pro-inflammatory cytokines/chemokines compared to GMMA with wild-type lipid A. Intracellular cytokine staining indicated monocytes and dendritic cells as the main source of the cytokines produced. Overall, these data provide new insights into the activation of key immune cells potentially targeted by GMMA-based vaccines.
Keywords: GMMA; OMV; Shigella sonnei; hPBMCs; immune response; in vitro; vaccines.
Copyright © 2022 Tondi, Clemente, Esposito, Sammicheli, Tavarini, Martin, Rossi, Micoli, Bartolini, Brazzoli, Ulivieri, Blohmke and Schiavetti.
Conflict of interest statement
STo is a student at the University of Siena and participated in a post graduate studentship program at GSK. BC, CE, CS, STa, MB, CB, and FS are employees of GSK group of companies. OR, FM, and LM are employees of the GSK Vaccines Institute for Global Health Srl, an affiliate of GlaxoSmithKline Biologicals SA. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. This work was sponsored by GlaxoSmithKline Biologicals SA which was involved in all stages of the study conduct and analysis.
Figures
Similar articles
-
Molecular Signature of Monocytes Shaped by the Shigella sonnei 1790-Generalized Modules for Membrane Antigens Vaccine.Int J Mol Sci. 2024 Jan 17;25(2):1116. doi: 10.3390/ijms25021116. Int J Mol Sci. 2024. PMID: 38256189 Free PMC article.
-
Modulation of endotoxicity of Shigella generalized modules for membrane antigens (GMMA) by genetic lipid A modifications: relative activation of TLR4 and TLR2 pathways in different mutants.J Biol Chem. 2014 Sep 5;289(36):24922-35. doi: 10.1074/jbc.M114.566570. Epub 2014 Jul 14. J Biol Chem. 2014. PMID: 25023285 Free PMC article.
-
Production of a Shigella sonnei Vaccine Based on Generalized Modules for Membrane Antigens (GMMA), 1790GAHB.PLoS One. 2015 Aug 6;10(8):e0134478. doi: 10.1371/journal.pone.0134478. eCollection 2015. PLoS One. 2015. PMID: 26248044 Free PMC article.
-
GMMA-Based Vaccines: The Known and The Unknown.Front Immunol. 2021 Aug 3;12:715393. doi: 10.3389/fimmu.2021.715393. eCollection 2021. Front Immunol. 2021. PMID: 34413858 Free PMC article. Review.
-
Towards a Four-Component GMMA-Based Vaccine against Shigella.Vaccines (Basel). 2022 Feb 18;10(2):328. doi: 10.3390/vaccines10020328. Vaccines (Basel). 2022. PMID: 35214786 Free PMC article. Review.
Cited by
-
Exploring the Role of GMMA Components in the Immunogenicity of a 4-Valent Vaccine against Shigella.Int J Mol Sci. 2023 Feb 1;24(3):2742. doi: 10.3390/ijms24032742. Int J Mol Sci. 2023. PMID: 36769063 Free PMC article.
-
Molecular Signature of Monocytes Shaped by the Shigella sonnei 1790-Generalized Modules for Membrane Antigens Vaccine.Int J Mol Sci. 2024 Jan 17;25(2):1116. doi: 10.3390/ijms25021116. Int J Mol Sci. 2024. PMID: 38256189 Free PMC article.
-
Outer Membrane Vesicle Vaccine Platforms.BioDrugs. 2024 Jan;38(1):47-59. doi: 10.1007/s40259-023-00627-0. Epub 2023 Oct 5. BioDrugs. 2024. PMID: 37796436 Free PMC article. Review.
References
-
- Benjamini Y., Hochberg Y. J. (1995). Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B Methodol. 57 (1), 289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

