Myricanol Inhibits Platelet Derived Growth Factor-BB-Induced Vascular Smooth Muscle Cells Proliferation and Migration in vitro and Intimal Hyperplasia in vivo by Targeting the Platelet-Derived Growth Factor Receptor-β and NF-κB Signaling
- PMID: 35185599
- PMCID: PMC8850918
- DOI: 10.3389/fphys.2021.790345
Myricanol Inhibits Platelet Derived Growth Factor-BB-Induced Vascular Smooth Muscle Cells Proliferation and Migration in vitro and Intimal Hyperplasia in vivo by Targeting the Platelet-Derived Growth Factor Receptor-β and NF-κB Signaling
Abstract
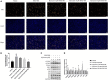
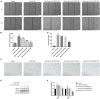
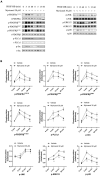
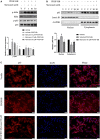
The abnormal proliferation and migration of Vascular smooth muscle cells (VSMCs) are related to many cardiovascular diseases, including atherosclerosis, restenosis after balloon angioplasty, hypertension, etc. Myricanol is a diarylheptanoid that can be separated from the bark of Myrica rubra. It has been reported that myricanol can anti-inflammatory, anti-cancer, anti-neurodegenerative, promote autophagic clearance of tau and prevent muscle atrophy. But its potential role in the cardiovascular field remains unknown. In this study, we investigated the effect of myricanol on the proliferation and migration of VSMCs in vitro and on the intimal hyperplasia in vivo. In vitro experiments, we found myricanol can inhibit the proliferation and migration of VSMCs induced by PDGF-BB. In terms of mechanism, the preincubation of myricanol can suppress the PDGF-BB induced phosphorylation of PDGFRβ and its downstream such as PLCγ1, Src, and MAPKs. In addition, NF-kB p65 translocation was also suppressed by myricanol. In vivo experiments, we found myricanol can suppress the intimal hyperplasia after wire ligation of the carotid artery in mice. These results may provide a new strategy for the prevention and treatment of coronary atherosclerosis and post-stent stenosis in the future.
Keywords: PDGF-BB; PDGFRβ; intimal hyperplasia; myricanol; vascular smooth muscle cells.
Copyright © 2022 Fan, Wang, Huang and Liang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Ruxolitinib attenuates intimal hyperplasia via inhibiting JAK2/STAT3 signaling pathway activation induced by PDGF-BB in vascular smooth muscle cells.Microvasc Res. 2020 Nov;132:104060. doi: 10.1016/j.mvr.2020.104060. Epub 2020 Aug 18. Microvasc Res. 2020. PMID: 32818511
-
Suppressive effect of formononetin on platelet-derived growth factor-BB-stimulated proliferation and migration of vascular smooth muscle cells.Exp Ther Med. 2016 Sep;12(3):1901-1907. doi: 10.3892/etm.2016.3514. Epub 2016 Jul 13. Exp Ther Med. 2016. PMID: 27588108 Free PMC article.
-
Chicoric acid prevents PDGF-BB-induced VSMC dedifferentiation, proliferation and migration by suppressing ROS/NFκB/mTOR/P70S6K signaling cascade.Redox Biol. 2018 Apr;14:656-668. doi: 10.1016/j.redox.2017.11.012. Epub 2017 Nov 16. Redox Biol. 2018. PMID: 29175753 Free PMC article.
-
MiR-7-5p attenuates vascular smooth muscle cell migration and intimal hyperplasia after vascular injury by NF-kB signaling.Biochem Biophys Rep. 2022 Dec 31;33:101394. doi: 10.1016/j.bbrep.2022.101394. eCollection 2023 Mar. Biochem Biophys Rep. 2022. PMID: 36601516 Free PMC article.
-
MicroRNA-451 inhibits vascular smooth muscle cell migration and intimal hyperplasia after vascular injury via Ywhaz/p38 MAPK pathway.Exp Cell Res. 2019 Jun 15;379(2):214-224. doi: 10.1016/j.yexcr.2019.03.033. Epub 2019 Mar 29. Exp Cell Res. 2019. PMID: 30930138
Cited by
-
Frequency-dependent signaling in cardiac myocytes.Front Physiol. 2022 Sep 2;13:926422. doi: 10.3389/fphys.2022.926422. eCollection 2022. Front Physiol. 2022. PMID: 36117711 Free PMC article.
-
Myricanol attenuates sepsis-induced inflammatory responses by nuclear factor erythroid 2-related factor 2 signaling and nuclear factor kappa B/mitogen-activated protein kinase pathway via upregulating Sirtuin 1.Inflammopharmacology. 2024 Jun;32(3):1887-1901. doi: 10.1007/s10787-024-01448-5. Epub 2024 Mar 25. Inflammopharmacology. 2024. PMID: 38526770
References
LinkOut - more resources
Full Text Sources
Miscellaneous

