Phosphorylation of BCL2 at the Ser70 site mediates RANKL-induced osteoclast precursor autophagy and osteoclastogenesis
- PMID: 35183115
- PMCID: PMC8858497
- DOI: 10.1186/s10020-022-00449-w
Phosphorylation of BCL2 at the Ser70 site mediates RANKL-induced osteoclast precursor autophagy and osteoclastogenesis
Abstract
Background: Phosphorylation modification of BCL2 is involved in receptor activator of nuclear factor-κB ligand (RANKL)-induced autophagy of osteoclast precursors (OCPs) and osteoclastogenesis. As an antiapoptotic molecule, the role of BCL2 phosphorylation in osteoclastogenesis is unknown. This study aimed to explore how BCL2 phosphorylation at specific sites regulates osteoclastogenesis.
Methods: We first examined the effects of RANKL on BCL2 phosphorylation at different sites (Ser70 and Ser87) in OCPs. In vivo, transgenic mice overexpressing RANKL (Tg-hRANKL mice) were used to observe the effects of RANKL on phosphorylated BCL2 at different sites in OCPs of trabecular bone. Subsequently, using site-directed mutagenesis, we observed the respective effect of BCL2 mutations at different phosphorylation sites in OCPs on osteoclastogenesis, apoptosis, autophagy and the affinity between BCL2 and Beclin1/BAX under RANKL intervention.
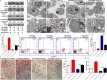
Results: RANKL promoted BCL2 phosphorylation at the Ser70 (S70) site, but not the Ser87 (S87) site, in OCPs. Moreover, Tg-hRANKL mice had stronger BCL2 phosphorylation capacity at S70, not S87, in the OCPs of trabecular bone than wild-type mice in the same nest. Furthermore, BCL2 mutation at S70, not S87, inhibited RANKL-induced osteoclast differentiation and bone resorption activity. In addition, BCL2 mutation at S70 promoted OCP apoptosis, while BCL2 mutation at S87 showed the opposite effect. Remarkably, the BCL2 mutation at S70, not S87, inhibited OCP autophagic activity. Furthermore, BCL2 mutation at S70 enhanced the coimmunoprecipitation of BCL2 and Beclin1, whereas BCL2 mutation at S87 enhanced the coimmunoprecipitation of BCL2 and BAX in OCPs. More importantly, OCP autophagy, osteoclast differentiation and resorption pits inhibited by BCL2 mutation at S70 could be reversed by Beclin1 upregulation with TAT-Beclin1.
Conclusion: RANKL activates OCP autophagy through BCL2 phosphorylation at S70, thereby promoting osteoclastogenesis, which indicates that the inactivation of BCL2 at S70 in OCPs may be a therapeutic strategy for pathological bone loss.
Keywords: Autophagy; BCL2 phosphorylation; Osteoclast; RANKL; Ser70; Ser87.
© 2022. The Author(s).
Conflict of interest statement
The authors have no competing interests to disclose.
Figures






Similar articles
-
Curcumin suppresses RANKL-induced osteoclast precursor autophagy in osteoclastogenesis by inhibiting RANK signaling and downstream JNK-BCL2-Beclin1 pathway.Biomed J. 2024 Feb;47(1):100605. doi: 10.1016/j.bj.2023.100605. Epub 2023 May 11. Biomed J. 2024. PMID: 37179010 Free PMC article.
-
JNK1 regulates RANKL-induced osteoclastogenesis via activation of a novel Bcl-2-Beclin1-autophagy pathway.FASEB J. 2019 Oct;33(10):11082-11095. doi: 10.1096/fj.201802597RR. Epub 2019 Jul 11. FASEB J. 2019. PMID: 31295022
-
1α,25-(OH)2-D3 promotes the autophagy during osteoclastogenesis by enhancing RANKL-RANK-TRAF6 signaling.In Vitro Cell Dev Biol Anim. 2021 Oct;57(9):878-885. doi: 10.1007/s11626-021-00632-z. Epub 2021 Nov 15. In Vitro Cell Dev Biol Anim. 2021. PMID: 34780049
-
IL-17A inhibits the degradation of RANKL in osteoblasts by inhibiting BCL2-Beclin1-autophagy signaling.In Vitro Cell Dev Biol Anim. 2023 Apr;59(4):300-311. doi: 10.1007/s11626-023-00761-7. Epub 2023 Mar 31. In Vitro Cell Dev Biol Anim. 2023. PMID: 37002492
-
Osteoclast differentiation by RANKL and OPG signaling pathways.J Bone Miner Metab. 2021 Jan;39(1):19-26. doi: 10.1007/s00774-020-01162-6. Epub 2020 Oct 20. J Bone Miner Metab. 2021. PMID: 33079279 Review.
Cited by
-
Role of O-linked N-acetylglucosamine protein modification in oxidative stress-induced autophagy: a novel target for bone remodeling.Cell Commun Signal. 2024 Jul 10;22(1):358. doi: 10.1186/s12964-024-01734-3. Cell Commun Signal. 2024. PMID: 38987770 Free PMC article. Review.
-
Regulatory mechanisms of autophagy-related ncRNAs in bone metabolic diseases.Front Pharmacol. 2023 Dec 7;14:1178310. doi: 10.3389/fphar.2023.1178310. eCollection 2023. Front Pharmacol. 2023. PMID: 38146458 Free PMC article. Review.
-
The Potential of Natural Compounds Regulating Autophagy in the Treatment of Osteoporosis.J Inflamm Res. 2023 Dec 8;16:6003-6021. doi: 10.2147/JIR.S437067. eCollection 2023. J Inflamm Res. 2023. PMID: 38088943 Free PMC article. Review.
-
IL-17A regulates autophagy and promotes osteoclast differentiation through the ERK/mTOR/Beclin1 pathway.PLoS One. 2023 Feb 16;18(2):e0281845. doi: 10.1371/journal.pone.0281845. eCollection 2023. PLoS One. 2023. PMID: 36795736 Free PMC article.
-
Melatonin alleviates palmitic acid-induced mitochondrial dysfunction by reducing oxidative stress and enhancing autophagy in bovine endometrial epithelial cells.J Anim Sci Biotechnol. 2024 Aug 8;15(1):108. doi: 10.1186/s40104-024-01064-x. J Anim Sci Biotechnol. 2024. PMID: 39113148 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

