Strategies to control therapeutic antibody glycosylation during bioprocessing: Synthesis and separation
- PMID: 35182428
- PMCID: PMC9310845
- DOI: 10.1002/bit.28066
Strategies to control therapeutic antibody glycosylation during bioprocessing: Synthesis and separation
Abstract
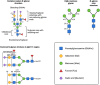
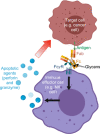
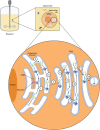
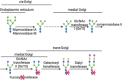
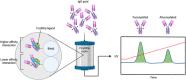
Glycosylation can be a critical quality attribute in biologic manufacturing. In particular, it has implications on the half-life, immunogenicity, and pharmacokinetics of therapeutic monoclonal antibodies (mAbs), and must be closely monitored throughout drug development and manufacturing. To address this, advances have been made primarily in upstream processing, including mammalian cell line engineering, to yield more predictably glycosylated mAbs and the addition of media supplements during fermentation to manipulate the metabolic pathways involved in glycosylation. A more robust approach would be a conjoined upstream-downstream processing strategy. This could include implementing novel downstream technologies, such as the use of Fc γ-based affinity ligands for the separation of mAb glycovariants. This review highlights the importance of controlling therapeutic antibody glycosylation patterns, the challenges faced in terms of glycosylation during mAb biosimilar development, current efforts both upstream and downstream to control glycosylation and their limitations, and the need for research in the downstream space to establish holistic and consistent manufacturing processes for the production of antibody therapies.
Keywords: CQAs; N-glycosylation; antibodies; biosimilars; downstream glycosylation bioprocessing; mAbs.
© 2022 The Authors. Biotechnology and Bioengineering published by Wiley Periodicals LLC.
Figures
Similar articles
-
Need for a risk-based control strategy for managing glycosylation profile for biosimilar products.Expert Opin Biol Ther. 2022 Feb;22(2):123-131. doi: 10.1080/14712598.2021.1973425. Epub 2021 Sep 1. Expert Opin Biol Ther. 2022. PMID: 34431439
-
Glycosylation of biosimilars: Recent advances in analytical characterization and clinical implications.Anal Chim Acta. 2019 Dec 16;1089:1-18. doi: 10.1016/j.aca.2019.08.044. Epub 2019 Aug 22. Anal Chim Acta. 2019. PMID: 31627805 Review.
-
A rapid method for relative quantification of N-glycans from a therapeutic monoclonal antibody during trastuzumab biosimilar development.MAbs. 2020 Jan-Dec;12(1):1750794. doi: 10.1080/19420862.2020.1750794. MAbs. 2020. PMID: 32249667 Free PMC article.
-
Recent Advances in Biologic Therapeutic N-Glycan Preparation Techniques and Analytical Methods for Facilitating Biomanufacturing Automation.J Pharm Sci. 2023 Jun;112(6):1485-1491. doi: 10.1016/j.xphs.2023.01.012. Epub 2023 Jan 20. J Pharm Sci. 2023. PMID: 36682489 Review.
-
QbD-guided pharmaceutical development of Pembrolizumab biosimilar candidate PSG-024 propelled to industry meeting primary requirements of comparability to Keytruda®.Eur J Pharm Sci. 2022 Jun 1;173:106171. doi: 10.1016/j.ejps.2022.106171. Epub 2022 Apr 1. Eur J Pharm Sci. 2022. PMID: 35378209
Cited by
-
MiRNA Chaining for Efficient Stable Overexpression to Improve Protein Quantity and Quality in CHO Cells.Methods Mol Biol. 2025;2853:85-101. doi: 10.1007/978-1-0716-4104-0_7. Methods Mol Biol. 2025. PMID: 39460916
-
Glycoprotein In Vitro N-Glycan Processing Using Enzymes Expressed in E. coli.Molecules. 2023 Mar 18;28(6):2753. doi: 10.3390/molecules28062753. Molecules. 2023. PMID: 36985724 Free PMC article.
-
A comprehensive overview on antibody-drug conjugates: from the conceptualization to cancer therapy.Front Pharmacol. 2023 Sep 18;14:1274088. doi: 10.3389/fphar.2023.1274088. eCollection 2023. Front Pharmacol. 2023. PMID: 37790810 Free PMC article. Review.
-
Study on antibody Fc-glycosylation for optimal effector functions.Chem Commun (Camb). 2023 May 4;59(37):5555-5558. doi: 10.1039/d3cc00672g. Chem Commun (Camb). 2023. PMID: 37071468 Free PMC article.
-
Stable overexpression of native and artificial miRNAs for the production of differentially fucosylated antibodies in CHO cells.Eng Life Sci. 2024 Apr 1;24(6):2300234. doi: 10.1002/elsc.202300234. eCollection 2024 Jun. Eng Life Sci. 2024. PMID: 38845814 Free PMC article.
References
-
- Adamczyk, B. , Tharmalingam‐Jaikaran, T. , Schomberg, M. , Szekrényes, Á. , Kelly, R. M. , Karlsson, N. G. , & Rudd, P. M. (2014). Comparison of separation techniques for the elucidation of IgG N‐glycans pooled from healthy mammalian species. Carbohydrate Research, 389, 174–185. 10.1016/j.carres.2014.01.018 - DOI - PubMed
-
- Aghamohseni, H. , Ohadi, K. , Spearman, M. , Krahn, N. , Moo‐Young, M. , Scharer, J. M. , & Budman, H. M. (2014). Effects of nutrient levels and average culture pH on the glycosylation pattern of camelid‐humanized monoclonal antibody. Journal of Biotechnology, 186, 98–109. 10.1016/j.jbiotec.2014.05.024 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

