Metabolic effects of the schizophrenia-associated 3q29 deletion
- PMID: 35177588
- PMCID: PMC8854723
- DOI: 10.1038/s41398-022-01824-1
Metabolic effects of the schizophrenia-associated 3q29 deletion
Abstract
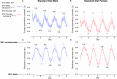
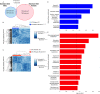
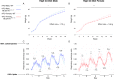
The 1.6 Mb 3q29 deletion is associated with developmental and psychiatric phenotypes, including a 40-fold increased risk for schizophrenia. Reduced birth weight and a high prevalence of feeding disorders in patients suggest underlying metabolic dysregulation. We investigated 3q29 deletion-induced metabolic changes using our previously generated heterozygous B6.Del16+/Bdh1-Tfrc mouse model. Animals were provided either standard chow (STD) or high-fat diet (HFD). Growth curves were performed on HFD mice to assess weight change (n = 30-50/group). Indirect calorimetry and untargeted metabolomics were performed on STD and HFD mice to evaluate metabolic phenotypes (n = 8-14/group). A behavioral battery was performed on STD and HFD mice to assess behavior change after the HFD challenge (n = 5-13/group). We found that B6.Del16+/Bdh1-Tfrc animals preferentially use dietary lipids as an energy source. Untargeted metabolomics of liver tissue showed a strong sex-dependent effect of the 3q29 deletion on fat metabolism. A HFD partially rescued the 3q29 deletion-associated weight deficit in females, but not males. Untargeted metabolomics of liver tissue after HFD revealed persistent fat metabolism alterations in females. The HFD did not affect B6.Del16+/Bdh1-Tfrc behavioral phenotypes, suggesting that 3q29 deletion-associated metabolic and behavioral outcomes are uncoupled. Our data suggest that dietary interventions to improve weight phenotypes in 3q29 deletion syndrome patients are unlikely to exacerbate behavioral manifestations. Our study also highlights the importance of assessing sex in metabolic studies and suggests that mechanisms underlying 3q29 deletion-associated metabolic phenotypes are sex-specific.
© 2022. The Author(s).
Conflict of interest statement
The authors report no competing interests.
Figures

Similar articles
-
Behavioral changes and growth deficits in a CRISPR engineered mouse model of the schizophrenia-associated 3q29 deletion.Mol Psychiatry. 2021 Mar;26(3):772-783. doi: 10.1038/s41380-019-0413-5. Epub 2019 Apr 11. Mol Psychiatry. 2021. PMID: 30976085 Free PMC article.
-
Psychiatric-disorder-related behavioral phenotypes and cortical hyperactivity in a mouse model of 3q29 deletion syndrome.Neuropsychopharmacology. 2019 Nov;44(12):2125-2135. doi: 10.1038/s41386-019-0441-5. Epub 2019 Jun 19. Neuropsychopharmacology. 2019. PMID: 31216562 Free PMC article.
-
Phenotypes for general behavior, activity, and body temperature in 3q29 deletion model mice.Transl Psychiatry. 2024 Mar 7;14(1):138. doi: 10.1038/s41398-023-02679-w. Transl Psychiatry. 2024. PMID: 38453903 Free PMC article.
-
A clinical case report and literature review of the 3q29 microdeletion syndrome.Clin Dysmorphol. 2015 Jul;24(3):89-94. doi: 10.1097/MCD.0000000000000077. Clin Dysmorphol. 2015. PMID: 25714563 Free PMC article. Review.
-
Unraveling the genetic architecture of copy number variants associated with schizophrenia and other neuropsychiatric disorders.J Neurosci Res. 2017 May;95(5):1144-1160. doi: 10.1002/jnr.23970. Epub 2016 Nov 8. J Neurosci Res. 2017. PMID: 27859486 Free PMC article. Review.
Cited by
-
Cross-species analysis identifies mitochondrial dysregulation as a functional consequence of the schizophrenia-associated 3q29 deletion.Sci Adv. 2023 Aug 18;9(33):eadh0558. doi: 10.1126/sciadv.adh0558. Epub 2023 Aug 16. Sci Adv. 2023. PMID: 37585521 Free PMC article.
-
Behavioral Phenotypes and Comorbidity in 3q29 Deletion Syndrome: Results from the 3q29 Registry.J Autism Dev Disord. 2024 Jan 12. doi: 10.1007/s10803-023-06218-w. Online ahead of print. J Autism Dev Disord. 2024. PMID: 38216835
-
17q12 deletion syndrome mouse model shows defects in craniofacial, brain and kidney development, and glucose homeostasis.Dis Model Mech. 2022 Dec 1;15(12):dmm049752. doi: 10.1242/dmm.049752. Epub 2022 Dec 13. Dis Model Mech. 2022. PMID: 36373506 Free PMC article.
References
-
- Akil M, Brewer GJ. Psychiatric and behavioral abnormalities in Wilson’s disease. Adv Neurol. 1995;65:171–8. - PubMed
-
- Cox DW. Disorders of copper transport. Br Med Bull. 1999;55:544–55. - PubMed
-
- Moghadasian MH. Cerebrotendinous xanthomatosis: clinical course, genotypes and metabolic backgrounds. Clin Invest Med. 2004;27:42–50. - PubMed
-
- Imrie J, Vijayaraghaven S, Whitehouse C, Harris S, Heptinstall L, Church H, et al. Niemann-Pick disease type C in adults. J Inherit Metab Dis. 2002;25:491–500. - PubMed
-
- Imrie J, Dasgupta S, Besley GT, Harris C, Heptinstall L, Knight S, et al. The natural history of Niemann-Pick disease type C in the UK. J Inherit Metab Dis. 2007;30:51–9. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

