Noncoding RNAs in Drug Resistance of Gastrointestinal Stromal Tumor
- PMID: 35174150
- PMCID: PMC8841737
- DOI: 10.3389/fcell.2022.808591
Noncoding RNAs in Drug Resistance of Gastrointestinal Stromal Tumor
Abstract
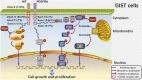
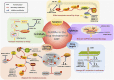
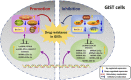
Gastrointestinal stromal tumor (GIST) is the most common mesenchymal tumor in the gastrointestinal tracts and a model for the targeted therapy of solid tumors because of the oncogenic driver mutations in KIT and PDGDRA genes, which could be effectively inhibited by the very first targeted agent, imatinib mesylate. Most of the GIST patients could benefit a lot from the targeted treatment of this receptor tyrosine kinase inhibitor. However, more than 50% of the patients developed resistance within 2 years after imatinib administration, limiting the long-term effect of imatinib. Noncoding RNAs (ncRNAs), the non-protein coding transcripts of human, were demonstrated to play pivotal roles in the resistance of various chemotherapy drugs. In this review, we summarized the mechanisms of how ncRNAs functioning on the drug resistance in GIST. During the drug resistance of GIST, there were five regulating mechanisms where the functions of ncRNAs concentrated: oxidative phosphorylation, autophagy, apoptosis, drug target changes, and some signaling pathways. Also, these effects of ncRNAs in drug resistance were divided into two aspects. How ncRNAs regulate drug resistance in GIST was further summarized according to ncRNA types, different drugs and categories of resistance. Moreover, clinical applications of these ncRNAs in GIST chemotherapies concentrated on the prognostic biomarkers and novel therapeutic targets.
Keywords: GIST; drug resistance; imatinib mesylate; noncoding RNAs; targeted therapies.
Copyright © 2022 Li, Guo, Sun and Fu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Imatinib mesylate: in the treatment of gastrointestinal stromal tumours.Drugs. 2003;63(5):513-22; discussion 523-4. doi: 10.2165/00003495-200363050-00005. Drugs. 2003. PMID: 12600228 Review.
-
Identification and treatment of chemoresistant inoperable or metastatic GIST: experience with the selective tyrosine kinase inhibitor imatinib mesylate (STI571).Eur J Cancer. 2002 Sep;38 Suppl 5:S52-9. doi: 10.1016/s0959-8049(02)80603-7. Eur J Cancer. 2002. PMID: 12528773 Review.
-
Targeting c-kit mutations in solid tumors: scientific rationale and novel therapeutic options.Semin Oncol. 2001 Oct;28(5 Suppl 17):19-26. Semin Oncol. 2001. PMID: 11740803 Review.
-
Crosstalk between KIT and FGFR3 Promotes Gastrointestinal Stromal Tumor Cell Growth and Drug Resistance.Cancer Res. 2015 Mar 1;75(5):880-91. doi: 10.1158/0008-5472.CAN-14-0573. Epub 2014 Nov 28. Cancer Res. 2015. PMID: 25432174 Free PMC article.
-
Dual Targeting of Insulin Receptor and KIT in Imatinib-Resistant Gastrointestinal Stromal Tumors.Cancer Res. 2017 Sep 15;77(18):5107-5117. doi: 10.1158/0008-5472.CAN-17-0917. Epub 2017 Jul 31. Cancer Res. 2017. PMID: 28760855
Cited by
-
Angiogenesis in gastrointestinal stromal tumors: From bench to bedside.World J Gastrointest Oncol. 2022 Aug 15;14(8):1469-1477. doi: 10.4251/wjgo.v14.i8.1469. World J Gastrointest Oncol. 2022. PMID: 36160752 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources

