Results of ARI-0001 CART19 Cells in Patients With Chronic Lymphocytic Leukemia and Richter's Transformation
- PMID: 35174095
- PMCID: PMC8841853
- DOI: 10.3389/fonc.2022.828471
Results of ARI-0001 CART19 Cells in Patients With Chronic Lymphocytic Leukemia and Richter's Transformation
Abstract
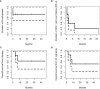
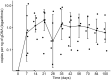
CART19 cells are emerging as an alternative therapy for patients with chronic lymphocytic leukemia (CLL). Here we report the outcome of nine consecutive patients with CLL treated with ARI-0001 CART19 cells, six of them with Richter's transformation (RT). One patient with RT never received therapy. The cytokine release syndrome rate was 87.5% (12.5% grade ≥3). Neurotoxicity was not observed in any patient. All patients experienced absolute B-cell aplasia, and seven (87.5%) responded to therapy. With a median follow-up of 5.6 months, two patients with RT experienced a CD19-negative relapse. In conclusion, ARI-0001 cell therapy was feasible, safe, and effective in patients with high-risk CLL or RT.
Keywords: CART; CD19; CLL; DLBCL; Richter disease.
Copyright © 2022 Ortiz-Maldonado, Frigola, Español-Rego, Balagué, Martínez-Cibrián, Magnano, Giné, Pascal, Correa, Martínez-Roca, Cid, Lozano, Villamor, Benítez-Ribas, Esteve, López-Guillermo, Campo, Urbano-Ispizua, Juan and Delgado.
Conflict of interest statement
VO-M: Consultant or advisory role (Kite Gilead, Celgene, Novartis), travel grants (Kite Gilead, Celgene, Novartis, Roche, Takeda, Janssen), honoraria (Kite Gilead). EG: Consultant or advisory role (Kite Gilead, Janssen, Genmab), research funding (Kite Gilead, Janssen, Roche). AM-R: Consultant or advisory role (Bristol Myers Squibb, Abbvie), travel grants (Kite Gilead, Roche, Takeda, Janssen, Abbvie), honoraria (Abbvie). ML: Honoraria (Grifols, Fresenius Kabi), research funding (Terumo BCT, Maco-Pharma). JE: Consultant or advisory role (Abbvie, Novartis, Celgene, Astellas, Jazz, Daiichi Dankyo, Roche, Amgen, Pfizer), travel grants (Celgene, Roche, Astellas, Daiichi Dankyo), research funding (Novartis, Celgene). AL-G: Consultant or advisory role (Roche, Kite Gilead, Celgene/Bristol-Myers, Incyte), honoraria (Roche, Novartis, Takeda, Bayer, Sandoz, Kern), research grants (Roche, Kite Gilead, Celgene/Bristol-Myers, Novartis, Incyte, Janssen, Pfizer, Takeda). ÁU-I: Consultant or advisory role (Kite Gilead, Celgene, Miltenyi), travel grants (Kite Gilead, Celgene). MJ: Consultant or advisory role (Kite Gilead, Grifols), honoraria (Kite Gilead, Grifols). The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Outcomes of Richter's transformation of chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL): an analysis of the SEER database.Ann Hematol. 2021 Oct;100(10):2513-2519. doi: 10.1007/s00277-021-04603-y. Epub 2021 Jul 19. Ann Hematol. 2021. PMID: 34279675
-
Detection of Richter's transformation of chronic lymphocytic leukemia by PET/CT.J Nucl Med. 2006 Aug;47(8):1267-73. J Nucl Med. 2006. PMID: 16883004
-
Risk factors associated with Richter's transformation in patients with chronic lymphocytic leukaemia: protocol for a retrospective population-based cohort study.BMJ Open. 2019 Mar 3;9(3):e023566. doi: 10.1136/bmjopen-2018-023566. BMJ Open. 2019. PMID: 30833314 Free PMC article.
-
Cellular Therapies in Chronic Lymphocytic Leukemia and Richter's Transformation: Recent Developments in Chimeric Antigen Receptor T-Cells, Natural Killer Cells, and Allogeneic Stem Cell Transplant.Cancers (Basel). 2023 Mar 18;15(6):1838. doi: 10.3390/cancers15061838. Cancers (Basel). 2023. PMID: 36980726 Free PMC article. Review.
-
Coexistence of chronic myeloid leukemia and diffuse large B-cell lymphoma with antecedent chronic lymphocytic leukemia: a case report and review of the literature.J Med Case Rep. 2018 Mar 11;12(1):64. doi: 10.1186/s13256-018-1612-4. J Med Case Rep. 2018. PMID: 29524963 Free PMC article. Review.
Cited by
-
Treatment of Richter's Transformation with Novel Therapies.Curr Hematol Malig Rep. 2024 Apr;19(2):45-55. doi: 10.1007/s11899-023-00721-8. Epub 2024 Jan 9. Curr Hematol Malig Rep. 2024. PMID: 38194201 Free PMC article. Review.
-
Response to therapy in Richter syndrome: a systematic review with meta-analysis of early clinical trials.Front Immunol. 2023 Nov 23;14:1295293. doi: 10.3389/fimmu.2023.1295293. eCollection 2023. Front Immunol. 2023. PMID: 38077330 Free PMC article.
-
Current and Future Perspectives for Chimeric Antigen Receptor T Cells Development in Poland.Biomedicines. 2022 Nov 13;10(11):2912. doi: 10.3390/biomedicines10112912. Biomedicines. 2022. PMID: 36428480 Free PMC article. Review.
-
CAR T-Based Therapies in Lymphoma: A Review of Current Practice and Perspectives.Biomedicines. 2022 Aug 12;10(8):1960. doi: 10.3390/biomedicines10081960. Biomedicines. 2022. PMID: 36009506 Free PMC article. Review.
-
Richter Transformation of Chronic Lymphocytic Leukemia-Are We Making Progress?Curr Hematol Malig Rep. 2023 Oct;18(5):144-157. doi: 10.1007/s11899-023-00701-y. Epub 2023 Jun 9. Curr Hematol Malig Rep. 2023. PMID: 37294394 Review.
References
-
- Kharfan-Dabaja MA, Kumar A, Hamadani M, Stilgenbauer S, Ghia P, Anasetti C, et al. . Clinical Practice Recommendations for Use of Allogeneic Hematopoietic Cell Transplantation in Chronic Lymphocytic Leukemia on Behalf of the Guidelines Committee of the American Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant (2016) 22(12):2117–25. doi: 10.1016/j.bbmt.2016.09.013 - DOI - PMC - PubMed
-
- Juan M, Delgado J, Calvo G, Trias E, Urbano-Ispizua A. Is Hospital Exemption an Alternative or a Bridge to European Medicines Agency for Developing Academic Chimeric Antigen Receptor T-Cell in Europe? Our Experience With ARI-0001. Hum Gene Ther (2021) 32(19–20):1004–7. doi: 10.1089/hum.2021.168 - DOI - PubMed
-
- Castella M, Boronat A, Martín-Ibáñez R, Rodríguez V, Suñé G, Caballero M, et al. . Development of a Novel Anti-CD19 Chimeric Antigen Receptor: A Paradigm for an Affordable CAR T Cell Production at Academic Institutions. Mol Ther Methods Clin Dev (2019) 12:134–44. doi: 10.1016/j.omtm.2018.11.010 - DOI - PMC - PubMed

