Treatment patterns, testing practices, and outcomes in the pre-FLAURA era for patients with EGFR mutation-positive advanced NSCLC: a retrospective chart review (REFLECT)
- PMID: 35173817
- PMCID: PMC8842149
- DOI: 10.1177/17588359211059874
Treatment patterns, testing practices, and outcomes in the pre-FLAURA era for patients with EGFR mutation-positive advanced NSCLC: a retrospective chart review (REFLECT)
Abstract
Introduction: For epidermal growth factor receptor mutation-positive (EGFRm) non-small-cell lung cancer (NSCLC), EGFR-tyrosine kinase inhibitors (EGFR-TKIs) are the preferred first-line (1 L) treatment in the advanced setting. Osimertinib, a third-generation EGFR-TKI, received full approval in 2017 for second-line (2 L) treatment of EGFR T790M-positive NSCLC. The REFLECT study characterizes real-world treatment/testing patterns, attrition rates, and outcomes in patients with EGFRm advanced NSCLC treated with 1 L first-/second-generation (1G/2G) EGFR-TKIs before 1 L osimertinib approval.
Methods: Retrospective chart review (NCT04031898) of European/Israeli adults with EGFRm unresectable locally advanced/metastatic NSCLC, initiating 1 L 1G/2G EGFR-TKIs 01/01/15-30/06/18 (index date).
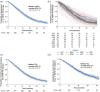
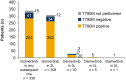
Results: In 896 patients (median follow-up of 21.5 months), the most frequently initiated 1 L EGFR-TKI was afatinib (45%). Disease progression was reported in 81%, including 10% (86/896) who died at 1 L. By the end of study, most patients discontinued 1 L (85%), of whom 33% did not receive 2 L therapy. From index, median 1 L real-world progression-free survival was 13.0 (95% confidence interval (CI): 12.3-14.1) months; median overall survival (OS) was 26.2 (95% CI: 23.6-28.4) months. 71% of patients with 1 L progression were tested for T790M; 58% were positive. Of those with T790M, 95% received osimertinib in 2 L or later. Central nervous system (CNS) metastases were recorded in 22% at index, and 15% developed CNS metastases during treatment (median time from index 13.5 months). Median OS was 19.4 months (95% CI: 17.1-22.1) in patients with CNS metastases at index, 24.8 months (95% CIs not available) with CNS metastases diagnosed during treatment, and 30.3 months (95% CI: 27.1, 33.8) with no CNS metastases recorded.
Conclusion: REFLECT is a large real-world study describing treatment patterns prior to 1 L osimertinib availability for EGFRm advanced NSCLC. Given the attrition rates highlighted in the study and the impact of CNS progression on outcomes, offering a 1 L EGFR-TKI with CNS penetration may improve patient outcomes in this treatment setting.
Keywords: EGFR T790M testing; EGFR-TKI; NSCLC; attrition; real-world evidence.
© The Author(s), 2021.
Conflict of interest statement
Conflict of interest statement: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: AA received honoraria from AstraZeneca, BMS, Eli Lilly, MSD, Pfizer, Roche and Takeda outside the submitted work. MH received honoraria for speaker’s bureau from AstraZeneca, BMS, Boehringer Ingelheim, MSD, Roche and Takeda, and served on advisory boards for AstraZeneca, BMS, Boehringer Ingelheim, MSD, Roche and Takeda. UJ served on advisory councils or committees for AstraZeneca, Boehringer Ingelheim, MSD and Roche, and received honoraria from AstraZeneca, BMS, Boehringer Ingelheim, MSD, Pfizer and Roche. ED served on advisory councils or committees for AstraZeneca, BMS, Novartis, Pfizer, Roche, Sanofi, and Takeda, received honoraria from AstraZeneca, BMS, MSD, Novartis, Pfizer, Roche, Sanofi, Pfizer, and Takeda and received consulting fees from AstraZeneca, BMS, Novartis, Pfizer, Roche, Sanofi, and Takeda, all outside the submitted work. AC served on advisory councils or committees for ASTRA, Boehringer Ingelheim and MSD, received honoraria from ASTRA, BMS, MSD and Roche and received consulting fees from ASTRA and Boehringer Ingelheim, outside the submitted work. AP has received personal fees and non-financial support from AstraZeneca, BMS, Boehringer Ingelheim, Pfizer and Roche, and personal fees from MSD, and Takeda outside the submitted work. TC served on advisory councils or committees for Amgen, Astellas, AstraZeneca, BMS, Boehringer Ingelheim Janssen, MSD. Roche, and Pfizer outside the submitted work. ISD has no disclosures to declare. JE and JA are employees of AstraZeneca. RO is an employee of AstraZeneca AG and owns stocks/shares in AstraZeneca AG. NP served on advisory councils or committees for AstraZeneca, Bayer, Boehringer Ingelheim, BMS, Eli Lilly, FoundationMedicine, Guardant360, Merck, MSD, Novartis, NovellusDx, Pfizer, Roche and Takeda, received honoraria and consulting fees from AstraZeneca, Bayer, Boehringer Ingelheim, BMS, Eli Lilly, FoundationMedicine, Guardant360, Merck, MSD, Novartis, NovellusDx, Pfizer, Roche and Takeda, and received grants or funds from AstraZeneca, Bayer, Boehringer Ingelheim, BMS, Eli Lilly, Merck, MSD, Novartis, Pfizer, Roche and Takeda.
Figures
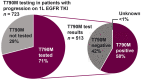
Similar articles
-
Treatment Patterns, Testing Practices, and Outcomes in Patients with EGFR Mutation-Positive Advanced Non-Small-Cell Lung Cancer in Poland: A Descriptive Analysis of National, Multicenter, Real-World Data from the REFLECT Study.Cancers (Basel). 2023 Mar 3;15(5):1581. doi: 10.3390/cancers15051581. Cancers (Basel). 2023. PMID: 36900371 Free PMC article.
-
Real-world outcomes, treatment patterns and T790M testing rates in non-small cell lung cancer patients treated with first-line first- or second-generation epidermal growth factor receptor tyrosine kinase inhibitors from the Slovenian cohort of the REFLECT study.Radiol Oncol. 2022 Aug 14;56(3):371-379. doi: 10.2478/raon-2022-0025. Radiol Oncol. 2022. PMID: 35853681 Free PMC article.
-
Real-World Pattern of Treatment and Clinical Outcomes of EGFR-Mutant Non-Small Cell Lung Cancer in a Single Academic Centre in Quebec.Curr Oncol. 2021 Dec 7;28(6):5179-5191. doi: 10.3390/curroncol28060434. Curr Oncol. 2021. PMID: 34940073 Free PMC article.
-
Ideal sequencing in Stage IV epidermal growth factor receptor mutant Non-Small-Cell Lung Cancer.Indian J Cancer. 2022 Mar;59(Supplement):S80-S89. doi: 10.4103/ijc.IJC_50_21. Indian J Cancer. 2022. PMID: 35343193 Review.
-
Osimertinib for EGFR-Mutant Non-Small-Cell Lung Cancer Central Nervous System Metastases: Current Evidence and Future Perspectives on Therapeutic Strategies.Target Oncol. 2023 Jan;18(1):9-24. doi: 10.1007/s11523-022-00941-7. Epub 2023 Jan 18. Target Oncol. 2023. PMID: 36652172 Review.
Cited by
-
Treatment Patterns, Testing Practices, and Outcomes in Patients with EGFR Mutation-Positive Advanced Non-Small-Cell Lung Cancer in Poland: A Descriptive Analysis of National, Multicenter, Real-World Data from the REFLECT Study.Cancers (Basel). 2023 Mar 3;15(5):1581. doi: 10.3390/cancers15051581. Cancers (Basel). 2023. PMID: 36900371 Free PMC article.
-
Cancer care treatment attrition in adults: Measurement approaches and inequities in patient dropout rates - a rapid review.BMC Cancer. 2024 Nov 1;24(1):1345. doi: 10.1186/s12885-024-13096-7. BMC Cancer. 2024. PMID: 39482591 Free PMC article. Review.
-
Real-World Testing Practices, Treatment Patterns and Clinical Outcomes in Patients from Central Eastern Europe with EGFR-Mutated Advanced Non-Small Cell Lung Cancer: A Retrospective Chart Review Study (REFLECT).Curr Oncol. 2022 Aug 17;29(8):5833-5845. doi: 10.3390/curroncol29080460. Curr Oncol. 2022. PMID: 36005198 Free PMC article.
-
Clinical Management of Patients with Non-Small Cell Lung Cancer, Brain Metastases, and Actionable Genomic Alterations: A Systematic Literature Review.Adv Ther. 2024 May;41(5):1815-1842. doi: 10.1007/s12325-024-02799-9. Epub 2024 Mar 21. Adv Ther. 2024. PMID: 38509433 Free PMC article. Review.
References
-
- Mok TS, Wu YL, Thongprasert S, et al.. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009; 361: 947–957. - PubMed
-
- Planchard D, Popat S, Kerr K, et al.. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2018; 29: iv192–iv237. - PubMed
-
- Hanna NH, Robinson AG, Temin S, et al.. Therapy for Stage IV non-small-cell lung cancer with driver alterations: ASCO and OH (CCO) joint guideline update. J Clin Oncol 2021; 39: 1040–1091. - PubMed
-
- Planchard D, Popat S, Kerr K, et al.. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Updated version published 15 September 2020 by the ESMO Guidelines Committee, https://www.esmo.org/guidelines/lung-and-chest-tumours/clinical-practice... (2020, accessed 25 September 2020).
-
- Sequist LV, Yang JC, Yamamoto N, et al.. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013; 31: 3327–3334. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

