Elevated Cytokine Levels in Plasma of Patients with SARS-CoV-2 Do Not Contribute to Pulmonary Microvascular Endothelial Permeability
- PMID: 35171047
- PMCID: PMC8849075
- DOI: 10.1128/spectrum.01671-21
Elevated Cytokine Levels in Plasma of Patients with SARS-CoV-2 Do Not Contribute to Pulmonary Microvascular Endothelial Permeability
Abstract
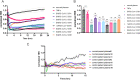
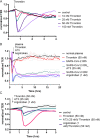
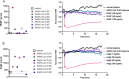
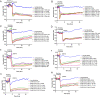
The vascular endothelial injury occurs in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections, but the mechanisms are poorly understood. We sought to determine the frequency and type of cytokine elevations and their relationship to endothelial injury induced by plasma from patients with SARS-CoV-2 versus controls. Plasma from eight consecutively enrolled patients hospitalized with acute SARS-CoV-2 infection was compared to controls. Endothelial cell (EC) barrier integrity was evaluated using ECIS (electric cell-substrate impedance sensing) on human lung microvascular EC. Plasma from all SARS-CoV-2 but none from controls decreased transendothelial resistance to a greater degree than that produced by tumor necrosis factor-alpha (TNF-α), the positive control for the assay. Thrombin, angiopoietin 2 (Ang2), and vascular endothelial growth factor (VEGF), complement factor C3a and C5a, and spike protein increased endothelial permeability, but to a lesser extent and a shorter duration when compared to SARS-CoV-2 plasma. Analysis of Ang2, VEGF, and 15 cytokines measured in plasma revealed striking patient-to-patient variability within the SARS-CoV-2 patients. Pretreatment with thrombin inhibitors, single, or combinations of neutralizing antibodies against cytokines, Ca3 and C5a receptor antagonists, or with ACE2 antibody failed to lessen the SARS-CoV-2 plasma-induced EC permeability. The EC barrier destructive effects of plasma from patients with SARS-CoV-2 were susceptible to heat inactivation. Plasma from patients hospitalized with acute SARS-CoV-2 infection uniformly disrupts lung microvascular integrity. No predicted single, or set of, cytokine(s) accounted for the enhanced vascular permeability, although the factor(s) were heat-labile. A still unidentified but potent circulating factor(s) appears to cause the EC disruption in SARS-CoV-2 infected patients. IMPORTANCE Lung vascular endothelial injury in SARS-CoV-2 patients is one of the most important causes of morbidity and mortality and has been linked to more severe complications including acute respiratory distress syndrome (ARDS) and subsequent death due to multiorgan failure. We have demonstrated that in eight consecutive patients with SARS-CoV-2, who were not selected for evidence of endothelial injury, the diluted plasma-induced intense lung microvascular damage, in vitro. Known endothelial barrier-disruptive agents and proposed mediators of increased endothelial permeability in SARS-CoV-2, induced changes in permeability that were smaller in magnitude and shorter in duration than plasma from patients with SARS-CoV-2. The effect on endothelial cell permeability of plasma from patients with SARS-CoV-2 was heat-labile. The main plasma factor that causes the increased endothelial permeability remains to be identified. Our study provides a possible approach for future studies to understand the underlying mechanisms leading to vascular injury in SARS-CoV-2 infections.
Keywords: ACE-2 receptor; SARS-CoV-2; SARS-CoV-2 plasma; antiinflammatory cytokines; barrier dysfunction; complements factors; cytokine; endothelial injury; endothelial permeability; heat inactivation; neutralizing antibodies; plasma; proinflammatory cytokines; spike protein.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

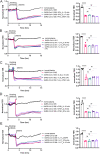
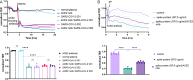
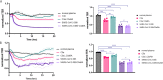
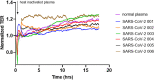
Similar articles
-
SARS-CoV-2 Spike Protein Destabilizes Microvascular Homeostasis.Microbiol Spectr. 2021 Dec 22;9(3):e0073521. doi: 10.1128/Spectrum.00735-21. Epub 2021 Dec 22. Microbiol Spectr. 2021. PMID: 34935423 Free PMC article.
-
The SARS-CoV-2 spike protein subunit S1 induces COVID-19-like acute lung injury in Κ18-hACE2 transgenic mice and barrier dysfunction in human endothelial cells.Am J Physiol Lung Cell Mol Physiol. 2021 Aug 1;321(2):L477-L484. doi: 10.1152/ajplung.00223.2021. Epub 2021 Jun 22. Am J Physiol Lung Cell Mol Physiol. 2021. PMID: 34156871 Free PMC article.
-
COVID-19-associated Lung Microvascular Endotheliopathy: A "From the Bench" Perspective.Am J Respir Crit Care Med. 2022 Oct 15;206(8):961-972. doi: 10.1164/rccm.202107-1774OC. Am J Respir Crit Care Med. 2022. PMID: 35649173 Free PMC article.
-
Endothelial cell dysfunction, coagulation, and angiogenesis in coronavirus disease 2019 (COVID-19).Microvasc Res. 2021 Sep;137:104188. doi: 10.1016/j.mvr.2021.104188. Epub 2021 May 20. Microvasc Res. 2021. PMID: 34022205 Free PMC article. Review.
-
COVID-19 is a systemic vascular hemopathy: insight for mechanistic and clinical aspects.Angiogenesis. 2021 Nov;24(4):755-788. doi: 10.1007/s10456-021-09805-6. Epub 2021 Jun 28. Angiogenesis. 2021. PMID: 34184164 Free PMC article. Review.
Cited by
-
Complement and COVID-19: Three years on, what we know, what we don't know, and what we ought to know.Immunobiology. 2023 May;228(3):152393. doi: 10.1016/j.imbio.2023.152393. Epub 2023 May 11. Immunobiology. 2023. PMID: 37187043 Free PMC article. Review.
-
C3a Mediates Endothelial Barrier Disruption in Brain-Derived, but Not Retinal, Human Endothelial Cells.Int J Mol Sci. 2024 Oct 19;25(20):11240. doi: 10.3390/ijms252011240. Int J Mol Sci. 2024. PMID: 39457022 Free PMC article.
-
Hydrogen Sulfide Ameliorates SARS-CoV-2-Associated Lung Endothelial Barrier Disruption.Biomedicines. 2023 Jun 22;11(7):1790. doi: 10.3390/biomedicines11071790. Biomedicines. 2023. PMID: 37509430 Free PMC article.
-
Direct endothelial ENaC activation mitigates vasculopathy induced by SARS-CoV2 spike protein.Front Immunol. 2023 Aug 10;14:1241448. doi: 10.3389/fimmu.2023.1241448. eCollection 2023. Front Immunol. 2023. PMID: 37638055 Free PMC article.
-
Plasma of COVID-19 Patients Does Not Alter Electrical Resistance of Human Endothelial Blood-Brain Barrier In Vitro.Function (Oxf). 2024 Jan 9;5(2):zqae002. doi: 10.1093/function/zqae002. eCollection 2024. Function (Oxf). 2024. PMID: 38486975 Free PMC article.
References
-
- World Health Organization. 2020. WHO coronavirus (COVID-19) dashboard. World Health Organization, Geneva, Switzerland. https://covid19.who.int/. Accessed December 12, 2021.
-
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. 2020. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395:497–506. doi:10.1016/S0140-6736(20)30183-5. - DOI - PMC - PubMed
-
- Flammer AJ, Anderson T, Celermajer DS, Creager MA, Deanfield J, Ganz P, Hamburg NM, Lüscher TF, Shechter M, Taddei S, Vita JA, Lerman A. 2012. The assessment of endothelial function: from research into clinical practice. Circulation 126:753–767. doi:10.1161/CIRCULATIONAHA.112.093245. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

