This is a preprint.
Boosting with Omicron-matched or historical mRNA vaccines increases neutralizing antibody responses and protection against B.1.1.529 infection in mice
- PMID: 35169795
- PMCID: PMC8845417
- DOI: 10.1101/2022.02.07.479419
Boosting with Omicron-matched or historical mRNA vaccines increases neutralizing antibody responses and protection against B.1.1.529 infection in mice
Abstract
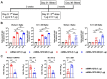
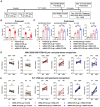
The B.1.1.529 Omicron variant jeopardizes vaccines designed with early pandemic spike antigens. Here, we evaluated in mice the protective activity of the Moderna mRNA-1273 vaccine against B.1.1.529 before or after boosting with preclinical mRNA-1273 or mRNA-1273.529, an Omicron-matched vaccine. Whereas two doses of mRNA-1273 vaccine induced high levels of serum neutralizing antibodies against historical WA1/2020 strains, levels were lower against B.1.1.529 and associated with infection and inflammation in the lung. A primary vaccination series with mRNA-1273.529 potently neutralized B.1.1.529 but showed limited inhibition of historical or other SARS-CoV-2 variants. However, boosting with mRNA-1273 or mRNA-1273.529 vaccines increased serum neutralizing titers and protection against B.1.1.529 infection. Nonetheless, the levels of inhibitory antibodies were higher, and viral burden and cytokines in the lung were slightly lower in mice given the Omicron-matched mRNA booster. Thus, in mice, boosting with mRNA-1273 or mRNA-1273.529 enhances protection against B.1.1.529 infection with limited differences in efficacy measured.
Figures
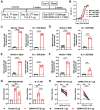
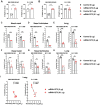
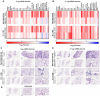
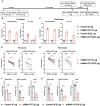

Similar articles
-
Boosting with variant-matched or historical mRNA vaccines protects against Omicron infection in mice.Cell. 2022 Apr 28;185(9):1572-1587.e11. doi: 10.1016/j.cell.2022.03.037. Epub 2022 Mar 28. Cell. 2022. PMID: 35452622 Free PMC article.
-
Antibody Avidity and Neutralizing Response against SARS-CoV-2 Omicron Variant after Infection or Vaccination.J Immunol Res. 2022 Aug 31;2022:4813199. doi: 10.1155/2022/4813199. eCollection 2022. J Immunol Res. 2022. PMID: 36093434 Free PMC article.
-
A Glycosylated RBD Protein Induces Enhanced Neutralizing Antibodies against Omicron and Other Variants with Improved Protection against SARS-CoV-2 Infection.J Virol. 2022 Sep 14;96(17):e0011822. doi: 10.1128/jvi.00118-22. Epub 2022 Aug 16. J Virol. 2022. PMID: 35972290 Free PMC article.
-
Boosting with Multiple Doses of mRNA Vaccine after Priming with Two Doses of Protein Subunit Vaccine MVC-COV1901 Elicited Robust Humoral and Cellular Immune Responses against Emerging SARS-CoV-2 Variants.Microbiol Spectr. 2022 Oct 26;10(5):e0060922. doi: 10.1128/spectrum.00609-22. Epub 2022 Aug 25. Microbiol Spectr. 2022. PMID: 36005765 Free PMC article.
-
mRNA Booster Vaccination Enhances Antibody Responses against SARS-CoV2 Omicron Variant in Individuals Primed with mRNA or Inactivated Virus Vaccines.Vaccines (Basel). 2022 Jun 30;10(7):1057. doi: 10.3390/vaccines10071057. Vaccines (Basel). 2022. PMID: 35891221 Free PMC article.
References
-
- Atmar R.L., Lyke K.E., Deming M.E., Jackson L.A., Branche A.R., El Sahly H.M., Rostad C.A., Martin J.M., Johnston C., Rupp R.E., et al. (2021). Heterologous SARS-CoV-2 Booster Vaccinations - Preliminary Report. medRxiv : the preprint server for health sciences. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
