Modeling Infection and Tropism of Human Parainfluenza Virus Type 3 in Ferrets
- PMID: 35164568
- PMCID: PMC8844927
- DOI: 10.1128/mbio.03831-21
Modeling Infection and Tropism of Human Parainfluenza Virus Type 3 in Ferrets
Abstract
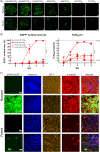
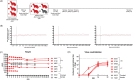
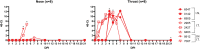
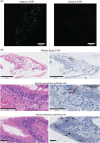
Human parainfluenza virus type 3 (HPIV-3) is a significant cause of lower respiratory tract infections, with the most severe disease in young infants, immunocompromised individuals, and the elderly. HPIV-3 infections are currently untreatable with licensed therapeutics, and prophylactic and therapeutic options are needed for patients at risk. To complement existing human airway models of HPIV-3 infection and develop an animal model to assess novel intervention strategies, we evaluated infection and transmission of HPIV-3 in ferrets. A well-characterized human clinical isolate (CI) of HPIV-3 engineered to express enhanced green fluorescent protein (rHPIV-3 CI-1-EGFP) was passaged on primary human airway epithelial cells (HAE) or airway organoids (AO) to avoid tissue culture adaptations. rHPIV3 CI-1-EGFP infection was assessed in vitro in ferret AO and in ferrets in vivo. Undifferentiated and differentiated ferret AO cultures supported rHPIV-3 CI-1-EGFP replication, but the ferret primary airway cells from AO were less susceptible and permissive than HAE. In vivo rHPIV-3 CI-1-EGFP replicated in the upper and lower airways of ferrets and targeted respiratory epithelial cells, olfactory epithelial cells, type I pneumocytes, and type II pneumocytes. The infection efficiently induced specific antibody responses. Taken together, ferrets are naturally susceptible to HPIV-3 infection; however, limited replication was observed that led to neither overt clinical signs nor ferret-to-ferret transmission. However, in combination with ferret AO, the ferret model of HPIV-3 infection, tissue tropism, and neutralizing antibodies complements human ex vivo lung models and can be used as a platform for prevention and treatment studies for this important respiratory pathogen. IMPORTANCE HPIV-3 is an important cause of pediatric disease and significantly impacts the elderly. Increasing numbers of immunocompromised patients suffer from HPIV-3 infections, often related to problems with viral clearance. There is a need to model HPIV-3 infections in vitro and in vivo to evaluate novel prophylaxis and treatment options. Currently existing animal models lack the potential for studying animal-to-animal transmission or the effect of immunosuppressive therapy. Here, we describe the use of the ferret model in combination with authentic clinical viruses to further complement human ex vivo models, providing a platform to study approaches to prevent and treat HPIV-3 infection. Although we did not detect ferret-to-ferret transmission in our studies, these studies lay the groundwork for further refinement of the ferret model to immunocompromised ferrets, allowing for studies of severe HPIV-3-associated disease. Such models for preclinical evaluation of prophylaxis and antivirals can contribute to reducing the global health burden of HPIV-3.
Keywords: animal models; parainfluenza virus; viral pathogenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Pathogenesis, Humoral Immune Responses, and Transmission between Cohoused Animals in a Ferret Model of Human Respiratory Syncytial Virus Infection.J Virol. 2018 Jan 30;92(4):e01322-17. doi: 10.1128/JVI.01322-17. Print 2018 Feb 15. J Virol. 2018. PMID: 29187546 Free PMC article.
-
Tropism and Infectivity of a Seasonal A(H1N1) and a Highly Pathogenic Avian A(H5N1) Influenza Virus in Primary Differentiated Ferret Nasal Epithelial Cell Cultures.J Virol. 2019 May 1;93(10):e00080-19. doi: 10.1128/JVI.00080-19. Print 2019 May 15. J Virol. 2019. PMID: 30814288 Free PMC article.
-
Viral Entry Properties Required for Fitness in Humans Are Lost through Rapid Genomic Change during Viral Isolation.mBio. 2018 Jul 3;9(4):e00898-18. doi: 10.1128/mBio.00898-18. mBio. 2018. PMID: 29970463 Free PMC article.
-
New antiviral approaches for human parainfluenza: Inhibiting the haemagglutinin-neuraminidase.Antiviral Res. 2019 Jul;167:89-97. doi: 10.1016/j.antiviral.2019.04.001. Epub 2019 Apr 3. Antiviral Res. 2019. PMID: 30951732 Review.
-
Parainfluenza Virus Infection.Semin Respir Crit Care Med. 2016 Aug;37(4):538-54. doi: 10.1055/s-0036-1584798. Epub 2016 Aug 3. Semin Respir Crit Care Med. 2016. PMID: 27486735 Free PMC article. Review.
Cited by
-
Organoid technology and applications in lung diseases: Models, mechanism research and therapy opportunities.Front Bioeng Biotechnol. 2022 Dec 8;10:1066869. doi: 10.3389/fbioe.2022.1066869. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36568297 Free PMC article. Review.
-
A robust mouse model of HPIV-3 infection and efficacy of GS-441524 against virus-induced lung pathology.Nat Commun. 2024 Sep 5;15(1):7765. doi: 10.1038/s41467-024-52071-5. Nat Commun. 2024. PMID: 39237507 Free PMC article.
-
Unnatural helical peptidic foldamers as protein segment mimics.Chem Soc Rev. 2023 Jul 31;52(15):4843-4877. doi: 10.1039/d2cs00395c. Chem Soc Rev. 2023. PMID: 37401344 Free PMC article. Review.
-
Analysis of gene expression dynamics and differential expression in viral infections using generalized linear models and quasi-likelihood methods.Front Microbiol. 2024 Apr 9;15:1342328. doi: 10.3389/fmicb.2024.1342328. eCollection 2024. Front Microbiol. 2024. PMID: 38655085 Free PMC article.
-
The relationship between autophagy and respiratory viruses.Arch Microbiol. 2024 Mar 4;206(4):136. doi: 10.1007/s00203-024-03838-3. Arch Microbiol. 2024. PMID: 38436746 Review.
References
-
- Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, O'Brien KL, Roca A, Wright PF, Bruce N, Chandran A, Theodoratou E, Sutanto A, Sedyaningsih ER, Ngama M, Munywoki PK, Kartasasmita C, Simões EA, Rudan I, Weber MW, Campbell H. 2010. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet (London, England) 375:1545–1555. doi:10.1016/S0140-6736(10)60206-1. - DOI - PMC - PubMed
-
- Wang X, Li Y, Deloria-Knoll M, Madhi SA, Cohen C, Arguelles VL, Basnet S, Bassat Q, Brooks WA, Echavarria M, Fasce RA, Gentile A, Goswami D, Homaira N, Howie SRC, Kotloff KL, Khuri-Bulos N, Krishnan A, Lucero MG, Lupisan S, Mathisen M, McLean KA, Mira-Iglesias A, Moraleda C, Okamoto M, Oshitani H, O'Brien KL, Owor BE, Rasmussen ZA, Rath BA, Salimi V, Sawatwong P, Scott JAG, Simões EAF, Sotomayor V, Thea DM, Treurnicht FK, Yoshida L-M, Zar HJ, Campbell H, Nair H. 2021. Global burden of acute lower respiratory infection associated with human parainfluenza virus in children younger than 5 years for 2018: a systematic review and meta-analysis. Lancet Glob Heal 9:e1077–e1087. doi:10.1016/S2214-109X(21)00218-7. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
