Exploring the Synergistic Anticancer Potential of Benzofuran-Oxadiazoles and Triazoles: Improved Ultrasound- and Microwave-Assisted Synthesis, Molecular Docking, Hemolytic, Thrombolytic and Anticancer Evaluation of Furan-Based Molecules
- PMID: 35164286
- PMCID: PMC8838991
- DOI: 10.3390/molecules27031023
Exploring the Synergistic Anticancer Potential of Benzofuran-Oxadiazoles and Triazoles: Improved Ultrasound- and Microwave-Assisted Synthesis, Molecular Docking, Hemolytic, Thrombolytic and Anticancer Evaluation of Furan-Based Molecules
Abstract
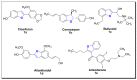
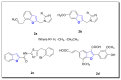
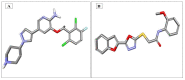
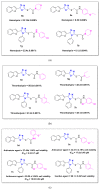
Ultrasound- and microwave-assisted green synthetic strategies were applied to furnish benzofuran-oxadiazole 5a-g and benzofuran-triazole 7a-h derivatives in good to excellent yields (60-96%), in comparison with conventional methods (36-80% yield). These synthesized derivatives were screened for hemolysis, thrombolysis and anticancer therapeutic potential against an A549 lung cancer cell line using an MTT assay. Derivatives 7b (0.1%) and 5e (0.5%) showed the least toxicity against RBCs. Hybrid 7f showed excellent thrombolysis activity (61.4%) when compared against reference ABTS. The highest anticancer activity was displayed by the 5d structural hybridwith cell viability 27.49 ± 1.90 and IC50 6.3 ± 0.7 μM values, which were considerably lower than the reference drug crizotinib (IC50 8.54 ± 0.84 μM). Conformational analysis revealed the spatial arrangement of compound 5d, which demonstrated its significant potency in comparison with crizotinib; therefore, scaffold 5d would be a promising anticancer agent on the basis of cytotoxicity studies, as well as in silico modeling studies.
Keywords: anticancer activities; benzofuran–oxadiazole; benzofuran–triazole; computational modeling; hemolytic activities; thrombolytic activities.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Synthesis, cytotoxicity, and molecular docking of substituted 3-(2-methylbenzofuran-3-yl)-5-(phenoxymethyl)-1,2,4-oxadiazoles.Arch Pharm (Weinheim). 2020 Jun;353(6):e2000006. doi: 10.1002/ardp.202000006. Epub 2020 Apr 20. Arch Pharm (Weinheim). 2020. PMID: 32309890
-
Synthesis and biological evaluation of 1-(benzofuran-3-yl)-4-(3,4,5-trimethoxyphenyl)-1H-1,2,3-triazole derivatives as tubulin polymerization inhibitors.Bioorg Chem. 2020 Jan;94:103392. doi: 10.1016/j.bioorg.2019.103392. Epub 2019 Oct 22. Bioorg Chem. 2020. PMID: 31669093
-
Synthesis, biological evaluation, and molecular docking studies of novel 1,3,4-oxadiazole derivatives possessing benzotriazole moiety as FAK inhibitors with anticancer activity.Bioorg Med Chem. 2013 Jul 1;21(13):3723-9. doi: 10.1016/j.bmc.2013.04.043. Epub 2013 Apr 23. Bioorg Med Chem. 2013. PMID: 23673215
-
Targeting the interplay between MMP-2, CA II and VEGFR-2 via new sulfonamide-tethered isomeric triazole hybrids; Microwave-assisted synthesis, computational studies and evaluation.Bioorg Chem. 2022 Jul;124:105816. doi: 10.1016/j.bioorg.2022.105816. Epub 2022 Apr 16. Bioorg Chem. 2022. PMID: 35489270 Review.
-
Green Synthetic Approach: An Efficient Eco-Friendly Tool for Synthesis of Biologically Active Oxadiazole Derivatives.Molecules. 2021 Feb 22;26(4):1163. doi: 10.3390/molecules26041163. Molecules. 2021. PMID: 33671751 Free PMC article. Review.
Cited by
-
Structure-Activity Relationship of Benzofuran Derivatives with Potential Anticancer Activity.Cancers (Basel). 2022 Apr 28;14(9):2196. doi: 10.3390/cancers14092196. Cancers (Basel). 2022. PMID: 35565325 Free PMC article. Review.
-
In Silico Development of Novel Benzofuran-1,3,4-Oxadiazoles as Lead Inhibitors of M. tuberculosis Polyketide Synthase 13.Pharmaceuticals (Basel). 2023 Jun 1;16(6):829. doi: 10.3390/ph16060829. Pharmaceuticals (Basel). 2023. PMID: 37375776 Free PMC article.
-
Unfolding the Antibacterial Activity and Acetylcholinesterase Inhibition Potential of Benzofuran-Triazole Hybrids: Synthesis, Antibacterial, Acetylcholinesterase Inhibition, and Molecular Docking Studies.Molecules. 2023 Aug 10;28(16):6007. doi: 10.3390/molecules28166007. Molecules. 2023. PMID: 37630258 Free PMC article.
-
An Exploration of the Inhibitory Mechanism of Rationally Screened Benzofuran-1,3,4-Oxadiazoles and-1,2,4-Triazoles as Inhibitors of NS5B RdRp Hepatitis C Virus through Pharmacoinformatic Approaches.Biomedicines. 2023 Nov 17;11(11):3085. doi: 10.3390/biomedicines11113085. Biomedicines. 2023. PMID: 38002085 Free PMC article.
-
Structure-Based Virtual Screening of Furan-1,3,4-Oxadiazole Tethered N-phenylacetamide Derivatives as Novel Class of hTYR and hTYRP1 Inhibitors.Pharmaceuticals (Basel). 2023 Feb 23;16(3):344. doi: 10.3390/ph16030344. Pharmaceuticals (Basel). 2023. PMID: 36986444 Free PMC article.
References
-
- Aslam S.N., Stevenson P.C., Phythian S.J., Veitch N.C., Hall D.R. Synthesis of cicerfuran, an antifungal benzofuran, and some related analogues. Tetrahedron. 2006;62:4214–4226. doi: 10.1016/j.tet.2006.02.015. - DOI
-
- Rangaswamy J., Kumar H.V., Harini S.T., Naik N. Functionalized 3-(benzofuran-2-yl)-5-(4-methoxyphenyl)-4,5-dihydro-1H-pyrazole scaffolds: A new class of antimicrobials and antioxidants. Arab. J. Chem. 2017;10:S2685–S2696. doi: 10.1016/j.arabjc.2013.10.012. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

