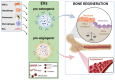
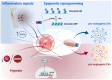
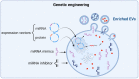
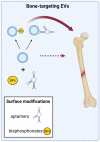
Educating EVs to Improve Bone Regeneration: Getting Closer to the Clinic
- PMID: 35163787
- PMCID: PMC8836395
- DOI: 10.3390/ijms23031865
Educating EVs to Improve Bone Regeneration: Getting Closer to the Clinic
Abstract
The incidence of bone-related disorders is continuously growing as the aging of the population in developing countries continues to increase. Although therapeutic interventions for bone regeneration exist, their effectiveness is questioned, especially under certain circumstances, such as critical size defects. This gap of curative options has led to the search for new and more effective therapeutic approaches for bone regeneration; among them, the possibility of using extracellular vesicles (EVs) is gaining ground. EVs are secreted, biocompatible, nano-sized vesicles that play a pivotal role as messengers between donor and target cells, mediated by their specific cargo. Evidence shows that bone-relevant cells secrete osteoanabolic EVs, whose functionality can be further improved by several strategies. This, together with the low immunogenicity of EVs and their storage advantages, make them attractive candidates for clinical prospects in bone regeneration. However, before EVs reach clinical translation, a number of concerns should be addressed. Unraveling the EVs' mode of action in bone regeneration is one of them; the molecular mediators driving their osteoanabolic effects in acceptor cells are now beginning to be uncovered. Increasing the functional and bone targeting abilities of EVs are also matters of intense research. Here, we summarize the cell sources offering osteoanabolic EVs, and the current knowledge about the molecular cargos that mediate bone regeneration. Moreover, we discuss strategies under development to improve the osteoanabolic and bone-targeting potential of EVs.
Keywords: MSCs; bone regeneration; extracellular vesicles; miRNAs; osteoanabolic.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Functionally engineered extracellular vesicles improve bone regeneration.Acta Biomater. 2020 Jun;109:182-194. doi: 10.1016/j.actbio.2020.04.017. Epub 2020 Apr 16. Acta Biomater. 2020. PMID: 32305445 Free PMC article.
-
Biomimetic synthesis and optimization of extracellular vesicles for bone regeneration.J Control Release. 2023 Mar;355:18-41. doi: 10.1016/j.jconrel.2023.01.057. Epub 2023 Feb 1. J Control Release. 2023. PMID: 36706840 Review.
-
Mesenchymal Stem Cell-Derived Extracellular Vesicles in Tissue Regeneration.Cell Transplant. 2020 Jan-Dec;29:963689720908500. doi: 10.1177/0963689720908500. Cell Transplant. 2020. PMID: 32207341 Free PMC article. Review.
-
Bone regeneration is mediated by macrophage extracellular vesicles.Bone. 2020 Dec;141:115627. doi: 10.1016/j.bone.2020.115627. Epub 2020 Sep 3. Bone. 2020. PMID: 32891867 Free PMC article.
-
Bone marrow stromal/stem cell-derived extracellular vesicles regulate osteoblast activity and differentiation in vitro and promote bone regeneration in vivo.Sci Rep. 2016 Feb 25;6:21961. doi: 10.1038/srep21961. Sci Rep. 2016. PMID: 26911789 Free PMC article.
Cited by
-
The Roles of Exosomal Proteins: Classification, Function, and Applications.Int J Mol Sci. 2023 Feb 4;24(4):3061. doi: 10.3390/ijms24043061. Int J Mol Sci. 2023. PMID: 36834471 Free PMC article. Review.
-
Epigenetic Reprogramming via Synergistic Hypomethylation and Hypoxia Enhances the Therapeutic Efficacy of Mesenchymal Stem Cell Extracellular Vesicles for Bone Repair.Int J Mol Sci. 2023 Apr 20;24(8):7564. doi: 10.3390/ijms24087564. Int J Mol Sci. 2023. PMID: 37108726 Free PMC article.
-
Bone marrow mesenchymal stem cells' osteogenic potential: superiority or non-superiority to other sources of mesenchymal stem cells?Cell Tissue Bank. 2023 Sep;24(3):663-681. doi: 10.1007/s10561-022-10066-w. Epub 2023 Jan 9. Cell Tissue Bank. 2023. PMID: 36622494 Review.
-
Bone marrow mesenchymal stem cell's exosomes as key nanoparticles in osteogenesis and bone regeneration: specific capacity based on cell type.Mol Biol Rep. 2022 Dec;49(12):12203-12218. doi: 10.1007/s11033-022-07807-1. Epub 2022 Oct 12. Mol Biol Rep. 2022. PMID: 36224447 Review.
-
Modifying MSCs-derived EVs with esterase-responsive and charge-reversal cationic polymers enhances bone regeneration.iScience. 2024 Aug 23;27(9):110801. doi: 10.1016/j.isci.2024.110801. eCollection 2024 Sep 20. iScience. 2024. PMID: 39310777 Free PMC article.
References
-
- Barnsley J., Buckland G., Chan P.E., Ong A., Ramos A.S., Baxter M., Laskou F., Dennison E.M., Cooper C., Patel H.P. Pathophysiology and treatment of osteoporosis: Challenges for clinical practice in older people. Aging Clin. Exp. Res. 2021;33:759–773. doi: 10.1007/s40520-021-01817-y. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

