Testing the Role of Glutamate NMDA Receptors in Peripheral Trigeminal Nociception Implicated in Migraine Pain
- PMID: 35163452
- PMCID: PMC8835926
- DOI: 10.3390/ijms23031529
Testing the Role of Glutamate NMDA Receptors in Peripheral Trigeminal Nociception Implicated in Migraine Pain
Abstract
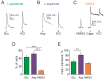
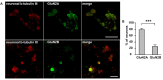
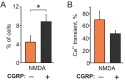
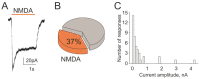
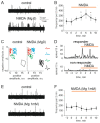
The pro-nociceptive role of glutamate in the CNS in migraine pathophysiology is well established. Glutamate, released from trigeminal afferents, activates second order nociceptive neurons in the brainstem. However, the function of peripheral glutamate receptors in the trigeminovascular system suggested as the origin site for migraine pain, is less known. In the current project, we used calcium imaging and patch clamp recordings from trigeminal ganglion (TG) neurons, immunolabelling, CGRP assay and direct electrophysiological recordings from rat meningeal afferents to investigate the role of glutamate in trigeminal nociception. Glutamate, aspartate, and, to a lesser extent, NMDA under free-magnesium conditions, evoked calcium transients in a fraction of isolated TG neurons, indicating functional expression of NMDA receptors. The fraction of NMDA sensitive neurons was increased by the migraine mediator CGRP. NMDA also activated slowly desensitizing currents in 37% of TG neurons. However, neither glutamate nor NMDA changed the level of extracellular CGRP. TG neurons expressed both GluN2A and GluN2B subunits of NMDA receptors. In addition, after removal of magnesium, NMDA activated persistent spiking activity in a fraction of trigeminal nerve fibers in meninges. Thus, glutamate activates NMDA receptors in somas of TG neurons and their meningeal nerve terminals in magnesium-dependent manner. These findings suggest that peripherally released glutamate can promote excitation of meningeal afferents implicated in generation of migraine pain in conditions of inherited or acquired reduced magnesium blockage of NMDA channels and support the usage of magnesium supplements in migraine.
Keywords: CGRP; NMDA; glutamate; migraine; trigeminal ganglia; trigeminal nerve.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

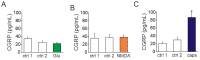
Similar articles
-
Serotonergic mechanisms of trigeminal meningeal nociception: Implications for migraine pain.Neuropharmacology. 2017 Apr;116:160-173. doi: 10.1016/j.neuropharm.2016.12.024. Epub 2016 Dec 23. Neuropharmacology. 2017. PMID: 28025094
-
Implications of high homocysteine levels in migraine pain: An experimental study of the excitability of peripheral meningeal afferents in rats with hyperhomocysteinemia.Headache. 2024 May;64(5):533-546. doi: 10.1111/head.14710. Epub 2024 Apr 22. Headache. 2024. PMID: 38650105
-
Mechanosensitive meningeal nociception via Piezo channels: Implications for pulsatile pain in migraine?Neuropharmacology. 2019 May 1;149:113-123. doi: 10.1016/j.neuropharm.2019.02.015. Epub 2019 Feb 13. Neuropharmacology. 2019. PMID: 30768945
-
CGRP and the Trigeminal System in Migraine.Headache. 2019 May;59(5):659-681. doi: 10.1111/head.13529. Epub 2019 Apr 14. Headache. 2019. PMID: 30982963 Free PMC article. Review.
-
Neuropeptide effects in the trigeminal system: pathophysiology and clinical relevance in migraine.Keio J Med. 2011;60(3):82-9. doi: 10.2302/kjm.60.82. Keio J Med. 2011. PMID: 21979827 Review.
Cited by
-
The Interactions of Magnesium Sulfate and Cromoglycate in a Rat Model of Orofacial Pain; The Role of Magnesium on Mast Cell Degranulation in Neuroinflammation.Int J Mol Sci. 2023 Mar 26;24(7):6241. doi: 10.3390/ijms24076241. Int J Mol Sci. 2023. PMID: 37047214 Free PMC article.
-
Role of ATP in migraine mechanisms: focus on P2X3 receptors.J Headache Pain. 2023 Jan 3;24(1):1. doi: 10.1186/s10194-022-01535-4. J Headache Pain. 2023. PMID: 36597043 Free PMC article. Review.
-
Targeting Peripheral N-Methyl-D-Aspartate Receptor (NMDAR): A Novel Strategy for the Treatment of Migraine.J Clin Med. 2023 Mar 10;12(6):2156. doi: 10.3390/jcm12062156. J Clin Med. 2023. PMID: 36983158 Free PMC article. Review.
-
Glia Signaling and Brain Microenvironment in Migraine.Mol Neurobiol. 2023 Jul;60(7):3911-3934. doi: 10.1007/s12035-023-03300-3. Epub 2023 Mar 30. Mol Neurobiol. 2023. PMID: 36995514 Review.
-
Arc-Mediated Synaptic Plasticity Regulates Cognitive Function in a Migraine Mouse Model.Brain Sci. 2023 Feb 15;13(2):331. doi: 10.3390/brainsci13020331. Brain Sci. 2023. PMID: 36831874 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

