circSLC41A1 Resists Porcine Granulosa Cell Apoptosis and Follicular Atresia by Promoting SRSF1 through miR-9820-5p Sponging
- PMID: 35163432
- PMCID: PMC8836210
- DOI: 10.3390/ijms23031509
circSLC41A1 Resists Porcine Granulosa Cell Apoptosis and Follicular Atresia by Promoting SRSF1 through miR-9820-5p Sponging
Abstract
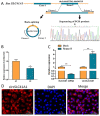
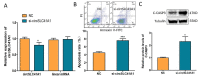
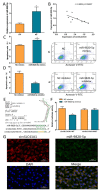
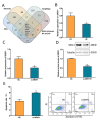
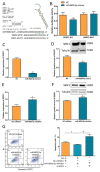
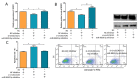
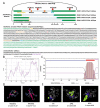
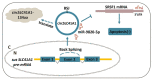
Ovarian granulosa cell (GC) apoptosis is the major cause of follicular atresia. Regulation of non-coding RNAs (ncRNAs) was proved to be involved in regulatory mechanisms of GC apoptosis. circRNAs have been recognized to play important roles in cellular activity. However, the regulatory network of circRNAs in follicular atresia has not been fully validated. In this study, we report a new circRNA, circSLC41A1, which has higher expression in healthy follicles compared to atretic follicles, and confirm its circular structure using RNase R treatment. The resistant function of circSLC41A1 during GC apoptosis was detected by si-RNA transfection and the competitive binding of miR-9820-5p by circSLC41A1 and SRSF1 was detected with a dual-luciferase reporter assay and co-transfection of their inhibitors or siRNA. Additionally, we predicted the protein-coding potential of circSLC41A1 and analyzed the structure of circSLC41A1-134aa. Our study revealed that circSLC41A1 enhanced SRSF1 expression through competitive binding of miR-9820-5p and demonstrated a circSLC41A1-miR-9820-5p-SRSF1 regulatory axis in follicular GC apoptosis. The study adds to knowledge of the post-transcriptional regulation of follicular atresia and provides insight into the protein-coding function of circRNA.
Keywords: SRSF1; ceRNA; circRNA; circSLC41A1; follicular atresia; granulosa cell apoptosis; miR-9820-5p; non-coding RNA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
CircINHA resists granulosa cell apoptosis by upregulating CTGF as a ceRNA of miR-10a-5p in pig ovarian follicles.Biochim Biophys Acta Gene Regul Mech. 2019 Oct;1862(10):194420. doi: 10.1016/j.bbagrm.2019.194420. Epub 2019 Aug 30. Biochim Biophys Acta Gene Regul Mech. 2019. PMID: 31476383
-
circRNA-Mediated Inhibin-Activin Balance Regulation in Ovarian Granulosa Cell Apoptosis and Follicular Atresia.Int J Mol Sci. 2021 Aug 24;22(17):9113. doi: 10.3390/ijms22179113. Int J Mol Sci. 2021. PMID: 34502034 Free PMC article.
-
miR-361-5p Mediates SMAD4 to Promote Porcine Granulosa Cell Apoptosis through VEGFA.Biomolecules. 2020 Sep 4;10(9):1281. doi: 10.3390/biom10091281. Biomolecules. 2020. PMID: 32899767 Free PMC article.
-
MicroRNAs in ovarian follicular atresia and granulosa cell apoptosis.Reprod Biol Endocrinol. 2019 Jan 10;17(1):9. doi: 10.1186/s12958-018-0450-y. Reprod Biol Endocrinol. 2019. PMID: 30630485 Free PMC article. Review.
-
Follicular atresia in pigs: measurement and physiology.J Anim Sci. 1995 Sep;73(9):2834-44. doi: 10.2527/1995.7392834x. J Anim Sci. 1995. PMID: 8582874 Review.
Cited by
-
Identification and Functional Analysis of circRNAs during Goat Follicular Development.Int J Mol Sci. 2024 Jul 9;25(14):7548. doi: 10.3390/ijms25147548. Int J Mol Sci. 2024. PMID: 39062792 Free PMC article.
-
SRSF1 is essential for primary follicle development by regulating granulosa cell survival via mRNA alternative splicing.Cell Mol Life Sci. 2023 Oct 31;80(11):343. doi: 10.1007/s00018-023-04979-2. Cell Mol Life Sci. 2023. PMID: 37907803 Free PMC article.
-
MEIS1 Is a Common Transcription Repressor of the miR-23a and NORHA Axis in Granulosa Cells.Int J Mol Sci. 2023 Feb 10;24(4):3589. doi: 10.3390/ijms24043589. Int J Mol Sci. 2023. PMID: 36834999 Free PMC article.
-
Comprehensive analysis of lncRNA-miRNA-mRNA ceRNA network and key genes in granulosa cells of patients with biochemical primary ovarian insufficiency.J Assist Reprod Genet. 2024 Jan;41(1):15-29. doi: 10.1007/s10815-023-02937-2. Epub 2023 Oct 17. J Assist Reprod Genet. 2024. PMID: 37847421 Free PMC article.
-
Sp1 transcription factor represses transcription of phosphatase and tensin homolog to aggravate lung injury in mice with type 2 diabetes mellitus-pulmonary tuberculosis.Bioengineered. 2022 Apr;13(4):9928-9944. doi: 10.1080/21655979.2022.2062196. Bioengineered. 2022. PMID: 35420971 Free PMC article.
References
-
- Schatten H., Constantinescu G.M. Animal Models and Human Reproduction. John Wiley & Sons Inc.; Hoboken, NJ, USA: 2017. pp. 213–214. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

