Cortical Dysplasia and the mTOR Pathway: How the Study of Human Brain Tissue Has Led to Insights into Epileptogenesis
- PMID: 35163267
- PMCID: PMC8835853
- DOI: 10.3390/ijms23031344
Cortical Dysplasia and the mTOR Pathway: How the Study of Human Brain Tissue Has Led to Insights into Epileptogenesis
Abstract
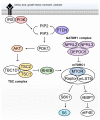
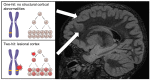
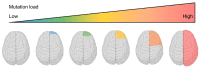
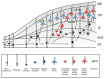
Type II focal cortical dysplasia (FCD) is a neuropathological entity characterised by cortical dyslamination with the presence of dysmorphic neurons only (FCDIIA) or the presence of both dysmorphic neurons and balloon cells (FCDIIB). The year 2021 marks the 50th anniversary of the recognition of FCD as a cause of drug resistant epilepsy, and it is now the most common reason for epilepsy surgery. The causes of FCD remained unknown until relatively recently. The study of resected human FCD tissue using novel genomic technologies has led to remarkable advances in understanding the genetic basis of FCD. Mechanistic parallels have emerged between these non-neoplastic lesions and neoplastic disorders of cell growth and differentiation, especially through perturbations of the mammalian target of rapamycin (mTOR) signalling pathway. This narrative review presents the advances through which the aetiology of FCDII has been elucidated in chronological order, from recognition of an association between FCD and the mTOR pathway to the identification of somatic mosaicism within FCD tissue. We discuss the role of a two-hit mechanism, highlight current challenges and future directions in detecting somatic mosaicism in brain and discuss how knowledge of FCD may inform novel precision treatments of these focal epileptogenic malformations of human cortical development.
Keywords: epilepsy; focal cortical dysplasia; genetics; hemimegalencephaly; mTOR signalling.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Somatic double-hit in MTOR and RPS6 in hemimegalencephaly with intractable epilepsy.Hum Mol Genet. 2019 Nov 15;28(22):3755-3765. doi: 10.1093/hmg/ddz194. Hum Mol Genet. 2019. PMID: 31411685 Free PMC article.
-
Review: Mechanistic target of rapamycin (mTOR) pathway, focal cortical dysplasia and epilepsy.Neuropathol Appl Neurobiol. 2018 Feb;44(1):6-17. doi: 10.1111/nan.12463. Neuropathol Appl Neurobiol. 2018. PMID: 29359340 Review.
-
FCD Type II and mTOR pathway: Evidence for different mechanisms involved in the pathogenesis of dysmorphic neurons.Epilepsy Res. 2017 Jan;129:146-156. doi: 10.1016/j.eplepsyres.2016.12.002. Epub 2016 Dec 7. Epilepsy Res. 2017. PMID: 28056425
-
Balloon cells promote immune system activation in focal cortical dysplasia type 2b.Neuropathol Appl Neurobiol. 2021 Oct;47(6):826-839. doi: 10.1111/nan.12736. Epub 2021 Jun 8. Neuropathol Appl Neurobiol. 2021. PMID: 34003514 Free PMC article.
-
mTOR pathway: Insights into an established pathway for brain mosaicism in epilepsy.Neurobiol Dis. 2023 Jun 15;182:106144. doi: 10.1016/j.nbd.2023.106144. Epub 2023 May 4. Neurobiol Dis. 2023. PMID: 37149062 Review.
Cited by
-
Anti-seizure gene therapy for focal cortical dysplasia.Brain. 2024 Feb 1;147(2):542-553. doi: 10.1093/brain/awad387. Brain. 2024. PMID: 38100333 Free PMC article.
-
mTOR Pathway Somatic Pathogenic Variants in Focal Malformations of Cortical Development: Novel Variants, Topographic Mapping, and Clinical Outcomes.Neurol Genet. 2023 Oct 26;9(6):e200103. doi: 10.1212/NXG.0000000000200103. eCollection 2023 Dec. Neurol Genet. 2023. PMID: 37900581 Free PMC article.
-
Neuropathology and epilepsy surgery: 2022 update.Free Neuropathol. 2022 May 3;3:3-12. doi: 10.17879/freeneuropathology-2022-3813. eCollection 2022 Jan. Free Neuropathol. 2022. PMID: 37284150 Free PMC article.
-
Further advances in epilepsy.J Neurol. 2023 Nov;270(11):5655-5670. doi: 10.1007/s00415-023-11860-6. Epub 2023 Jul 17. J Neurol. 2023. PMID: 37458794 Review.
-
Multi-tensor diffusion abnormalities of gray matter in an animal model of cortical dysplasia.Front Neurol. 2023 May 5;14:1124282. doi: 10.3389/fneur.2023.1124282. eCollection 2023. Front Neurol. 2023. PMID: 37342776 Free PMC article.
References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

