Apoptosis during ZIKA Virus Infection: Too Soon or Too Late?
- PMID: 35163212
- PMCID: PMC8835863
- DOI: 10.3390/ijms23031287
Apoptosis during ZIKA Virus Infection: Too Soon or Too Late?
Abstract
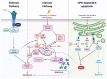
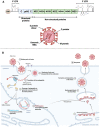
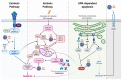
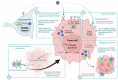
Cell death by apoptosis is a major cellular response in the control of tissue homeostasis and as a defense mechanism in the case of cellular aggression such as an infection. Cell self-destruction is part of antiviral responses, aimed at limiting the spread of a virus. Although it may contribute to the deleterious effects in infectious pathology, apoptosis remains a key mechanism for viral clearance and the resolution of infection. The control mechanisms of cell death processes by viruses have been extensively studied. Apoptosis can be triggered by different viral determinants through different pathways as a result of virally induced cell stresses and innate immune responses. Zika virus (ZIKV) induces Zika disease in humans, which has caused severe neurological forms, birth defects, and microcephaly in newborns during the last epidemics. ZIKV also surprised by revealing an ability to persist in the genital tract and in semen, thus being sexually transmitted. Mechanisms of diverting antiviral responses such as the interferon response, the role of cytopathic effects and apoptosis in the etiology of the disease have been widely studied and debated. In this review, we examined the interplay between ZIKV infection of different cell types and apoptosis and how the virus deals with this cellular response. We illustrate a duality in the effects of ZIKV-controlled apoptosis, depending on whether it occurs too early or too late, respectively, in neuropathogenesis, or in long-term viral persistence. We further discuss a prospective role for apoptosis in ZIKV-related therapies, and the use of ZIKV as an oncolytic agent.
Keywords: ZIKV; Zika virus; apoptosis; cell death.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
High-Throughput Screening Identifies Mixed-Lineage Kinase 3 as a Key Host Regulatory Factor in Zika Virus Infection.J Virol. 2019 Aug 28;93(18):e00758-19. doi: 10.1128/JVI.00758-19. Print 2019 Sep 15. J Virol. 2019. PMID: 31270223 Free PMC article.
-
Axl Promotes Zika Virus Entry and Modulates the Antiviral State of Human Sertoli Cells.mBio. 2019 Jul 16;10(4):e01372-19. doi: 10.1128/mBio.01372-19. mBio. 2019. PMID: 31311882 Free PMC article.
-
Zika Virus Induces Tumor Necrosis Factor-Related Apoptosis Inducing Ligand (TRAIL)-Mediated Apoptosis in Human Neural Progenitor Cells.Cells. 2020 Nov 16;9(11):2487. doi: 10.3390/cells9112487. Cells. 2020. PMID: 33207682 Free PMC article.
-
Advances in Zika Virus⁻Host Cell Interaction: Current Knowledge and Future Perspectives.Int J Mol Sci. 2019 Mar 4;20(5):1101. doi: 10.3390/ijms20051101. Int J Mol Sci. 2019. PMID: 30836648 Free PMC article. Review.
-
Probing Molecular Insights into Zika Virus⁻Host Interactions.Viruses. 2018 May 2;10(5):233. doi: 10.3390/v10050233. Viruses. 2018. PMID: 29724036 Free PMC article. Review.
Cited by
-
RNA-Seq analysis reveals the different mechanisms triggered by bovine and equine after infection with FMDV.Vet Med Sci. 2024 Sep;10(5):e1569. doi: 10.1002/vms3.1569. Vet Med Sci. 2024. PMID: 39287214 Free PMC article.
-
Discovery of New Zika Protease and Polymerase Inhibitors through the Open Science Collaboration Project OpenZika.J Chem Inf Model. 2022 Dec 26;62(24):6825-6843. doi: 10.1021/acs.jcim.2c00596. Epub 2022 Oct 14. J Chem Inf Model. 2022. PMID: 36239304 Free PMC article.
-
Concomitant pyroptotic and apoptotic cell death triggered in macrophages infected by Zika virus.PLoS One. 2022 Apr 21;17(4):e0257408. doi: 10.1371/journal.pone.0257408. eCollection 2022. PLoS One. 2022. PMID: 35446851 Free PMC article.
-
Orchestration of Mitochondrial Function and Remodeling by Post-Translational Modifications Provide Insight into Mechanisms of Viral Infection.Biomolecules. 2023 May 20;13(5):869. doi: 10.3390/biom13050869. Biomolecules. 2023. PMID: 37238738 Free PMC article. Review.
-
Distinct Replication Kinetics, Cytopathogenicity, and Immune Gene Regulation in Human Microglia Cells Infected with Asian and African Lineages of Zika Virus.Microorganisms. 2024 Sep 5;12(9):1840. doi: 10.3390/microorganisms12091840. Microorganisms. 2024. PMID: 39338514 Free PMC article.
References
-
- Krauer F., Riesen M., Reveiz L., Oladapo O.T., Martínez-Vega R., Porgo T.V., Haefliger A., Broutet N.J., Low N., Group W.Z.C.W. Zika Virus Infection as a Cause of Congenital Brain Abnormalities and Guillain–Barré Syndrome: Systematic Review. PLOS Med. 2017;14:e1002203. doi: 10.1371/journal.pmed.1002203. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

