Deubiquitinases in Neurodegeneration
- PMID: 35159365
- PMCID: PMC8834042
- DOI: 10.3390/cells11030556
Deubiquitinases in Neurodegeneration
Abstract
Ubiquitination refers to the conjugation of the ubiquitin protein (a small protein highly conserved among eukaryotes) to itself or to other proteins through differential use of ubiquitin's seven internal linkage sites or the amino-terminal amino group. By creating different chain lengths, an enormous proteomic diversity may be formed. This creates a signaling system that is central to controlling almost every conceivable protein function, from proteostasis to regulating enzyme function and everything in between. Protein ubiquitination is reversed through the activity of deubiquitinases (DUBs), enzymes that function to deconjugate ubiquitin from itself and protein substrates. DUBs are regulated through several mechanisms, from controlled subcellular localization within cells to developmental and tissue specific expression. Misregulation of DUBs has been implicated in several diseases including cancer and neurodegeneration. Here we present a brief overview of the role of DUBs in neurodegeneration, and as potential therapeutic targets.
Keywords: Drosophila; deubiquitinase; neurodegeneration; ubiquitin; ubiquitin-specific protease.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the writing of the manuscript, or in the decision to publish the review.
Figures
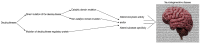
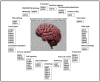
Similar articles
-
Dissenting degradation: Deubiquitinases in cell cycle and cancer.Semin Cancer Biol. 2020 Dec;67(Pt 2):145-158. doi: 10.1016/j.semcancer.2020.03.008. Epub 2020 Mar 20. Semin Cancer Biol. 2020. PMID: 32201366 Free PMC article. Review.
-
Structural basis for the linkage specificity of ubiquitin-binding domain and deubiquitinase.J Biochem. 2022 Jun 28;172(1):1-7. doi: 10.1093/jb/mvac031. J Biochem. 2022. PMID: 35394523 Review.
-
The role of deubiquitinases in breast cancer.Cancer Metastasis Rev. 2016 Dec;35(4):589-600. doi: 10.1007/s10555-016-9640-2. Cancer Metastasis Rev. 2016. PMID: 27844253 Free PMC article. Review.
-
Deubiquitinases: Modulators of Different Types of Regulated Cell Death.Int J Mol Sci. 2021 Apr 21;22(9):4352. doi: 10.3390/ijms22094352. Int J Mol Sci. 2021. PMID: 33919439 Free PMC article. Review.
-
Evaluating enzyme activities and structures of DUBs.Methods Enzymol. 2019;618:321-341. doi: 10.1016/bs.mie.2019.01.001. Epub 2019 Feb 22. Methods Enzymol. 2019. PMID: 30850058 Free PMC article.
Cited by
-
Pleiotropic Roles of a KEAP1-Associated Deubiquitinase, OTUD1.Antioxidants (Basel). 2023 Feb 1;12(2):350. doi: 10.3390/antiox12020350. Antioxidants (Basel). 2023. PMID: 36829909 Free PMC article. Review.
-
Identification of gene networks jointly associated with depressive symptoms and cardiovascular health metrics using whole blood transcriptome in the Young Finns Study.Front Psychiatry. 2024 Apr 25;15:1345159. doi: 10.3389/fpsyt.2024.1345159. eCollection 2024. Front Psychiatry. 2024. PMID: 38726387 Free PMC article.
-
Targeting Deubiquitinating Enzymes (DUBs) That Regulate Mitophagy via Direct or Indirect Interaction with Parkin.Int J Mol Sci. 2022 Oct 11;23(20):12105. doi: 10.3390/ijms232012105. Int J Mol Sci. 2022. PMID: 36292958 Free PMC article. Review.
-
USP8 promotes the tumorigenesis of intrahepatic cholangiocarcinoma via stabilizing OGT.Cancer Cell Int. 2024 Jul 7;24(1):238. doi: 10.1186/s12935-024-03370-w. Cancer Cell Int. 2024. PMID: 38973004 Free PMC article.
-
The emerging role and therapeutic implications of bacterial and parasitic deubiquitinating enzymes.Front Immunol. 2023 Nov 22;14:1303072. doi: 10.3389/fimmu.2023.1303072. eCollection 2023. Front Immunol. 2023. PMID: 38077335 Free PMC article. Review.
References
-
- Pirone L., Xolalpa W., Sigurðsson J.O., Ramirez J., Pérez C., Gonzalez M., de Sabando A.R., Elortza F., Rodriguez M.S., Mayor U., et al. A comprehensive platform for the analysis of ubiquitin-like protein modifications using in vivo biotinylation. Sci. Rep. 2017;7:40756. doi: 10.1038/srep40756. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

