Beta-Amyloid Instigates Dysfunction of Mitochondria in Cardiac Cells
- PMID: 35159183
- PMCID: PMC8834545
- DOI: 10.3390/cells11030373
Beta-Amyloid Instigates Dysfunction of Mitochondria in Cardiac Cells
Abstract
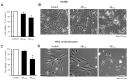
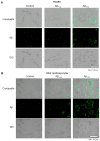
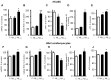
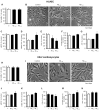
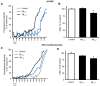
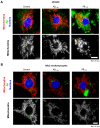
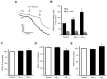
Alzheimer's disease (AD) includes the formation of extracellular deposits comprising aggregated β-amyloid (Aβ) fibers associated with oxidative stress, inflammation, mitochondrial abnormalities, and neuronal loss. There is an associative link between AD and cardiac diseases; however, the mechanisms underlying the potential role of AD, particularly Aβ in cardiac cells, remain unknown. Here, we investigated the role of mitochondria in mediating the effects of Aβ1-40 and Aβ1-42 in cultured cardiomyocytes and primary coronary endothelial cells. Our results demonstrated that Aβ1-40 and Aβ1-42 are differently accumulated in cardiomyocytes and coronary endothelial cells. Aβ1-42 had more adverse effects than Aβ1-40 on cell viability and mitochondrial function in both types of cells. Mitochondrial and cellular ROS were significantly increased, whereas mitochondrial membrane potential and calcium retention capacity decreased in both types of cells in response to Aβ1-42. Mitochondrial dysfunction induced by Aβ was associated with apoptosis of the cells. The effects of Aβ1-42 on mitochondria and cell death were more evident in coronary endothelial cells. In addition, Aβ1-40 and Aβ1-42 significantly increased Ca2+ -induced swelling in mitochondria isolated from the intact rat hearts. In conclusion, this study demonstrates the toxic effects of Aβ on cell survival and mitochondria function in cardiac cells.
Keywords: Alzheimer’s disease; beta-amyloid; cardiomyocytes; coronary artery endothelial cells; mitochondria.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
The Effects of Amyloid-β on Metabolomic Profiles of Cardiomyocytes and Coronary Endothelial Cells.J Alzheimers Dis. 2023;93(1):307-319. doi: 10.3233/JAD-221199. J Alzheimers Dis. 2023. PMID: 36970904 Free PMC article.
-
Activation of the endoplasmic reticulum stress response by the amyloid-beta 1-40 peptide in brain endothelial cells.Biochim Biophys Acta. 2013 Dec;1832(12):2191-203. doi: 10.1016/j.bbadis.2013.08.007. Epub 2013 Aug 28. Biochim Biophys Acta. 2013. PMID: 23994613
-
Site-specific glycation of Aβ1-42 affects fibril formation and is neurotoxic.J Biol Chem. 2019 May 31;294(22):8806-8818. doi: 10.1074/jbc.RA118.006846. Epub 2019 Apr 17. J Biol Chem. 2019. PMID: 30996005 Free PMC article.
-
Olesoxime improves cerebral mitochondrial dysfunction and enhances Aβ levels in preclinical models of Alzheimer's disease.Exp Neurol. 2020 Jul;329:113286. doi: 10.1016/j.expneurol.2020.113286. Epub 2020 Mar 18. Exp Neurol. 2020. PMID: 32199815 Review.
-
Synaptic Mitochondria: An Early Target of Amyloid-β and Tau in Alzheimer's Disease.J Alzheimers Dis. 2021;84(4):1391-1414. doi: 10.3233/JAD-215139. J Alzheimers Dis. 2021. PMID: 34719499 Review.
Cited by
-
Retinal Alterations Predict Early Prodromal Signs of Neurodegenerative Disease.Int J Mol Sci. 2024 Jan 30;25(3):1689. doi: 10.3390/ijms25031689. Int J Mol Sci. 2024. PMID: 38338966 Free PMC article. Review.
-
Autonomic nervous system and cardiac neuro-signaling pathway modulation in cardiovascular disorders and Alzheimer's disease.Front Physiol. 2023 Jan 30;14:1060666. doi: 10.3389/fphys.2023.1060666. eCollection 2023. Front Physiol. 2023. PMID: 36798942 Free PMC article. Review.
-
Amyloid beta 42 alters cardiac metabolism and impairs cardiac function in male mice with obesity.Nat Commun. 2024 Jan 15;15(1):258. doi: 10.1038/s41467-023-44520-4. Nat Commun. 2024. PMID: 38225272 Free PMC article.
-
Hypoxia-Inducible Factor-1α Protects Against Intervertebral Disc Degeneration Through Antagonizing Mitochondrial Oxidative Stress.Inflammation. 2023 Feb;46(1):270-284. doi: 10.1007/s10753-022-01732-y. Epub 2022 Sep 6. Inflammation. 2023. PMID: 36064808 Free PMC article.
-
Recent progresses in natural based therapeutic materials for Alzheimer's disease.Heliyon. 2024 Feb 15;10(4):e26351. doi: 10.1016/j.heliyon.2024.e26351. eCollection 2024 Feb 29. Heliyon. 2024. PMID: 38434059 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

