Gas Plasma Exposure of Glioblastoma Is Cytotoxic and Immunomodulatory in Patient-Derived GBM Tissue
- PMID: 35159079
- PMCID: PMC8834374
- DOI: 10.3390/cancers14030813
Gas Plasma Exposure of Glioblastoma Is Cytotoxic and Immunomodulatory in Patient-Derived GBM Tissue
Abstract
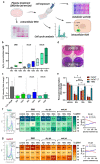
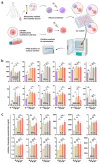
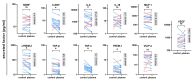
Glioblastoma multiforme (GBM) is the most common primary malignant adult brain tumor. Therapeutic options for glioblastoma are maximal surgical resection, chemotherapy, and radiotherapy. Therapy resistance and tumor recurrence demand, however, new strategies. Several experimental studies have suggested gas plasma technology, a partially ionized gas that generates a potent mixture of reactive oxygen species (ROS), as a future complement to the existing treatment arsenal. However, aspects such as immunomodulation, inflammatory consequences, and feasibility studies using GBM tissue have not been addressed so far. In vitro, gas plasma generated ROS that oxidized cells and led to a treatment time-dependent metabolic activity decline and G2 cell cycle arrest. In addition, peripheral blood-derived monocytes were co-cultured with glioblastoma cells, and immunomodulatory surface expression markers and cytokine release were screened. Gas plasma treatment of either cell type, for instance, decreased the expression of the M2-macrophage marker CD163 and the tolerogenic molecule SIGLEC1 (CD169). In patient-derived GBM tissue samples exposed to the plasma jet kINPen ex vivo, apoptosis was significantly increased. Quantitative chemokine/cytokine release screening revealed gas plasma exposure to significantly decrease 5 out of 11 tested chemokines and cytokines, namely IL-6, TGF-β, sTREM-2, b-NGF, and TNF-α involved in GBM apoptosis and immunomodulation. In summary, the immuno-modulatory and proapoptotic action shown in this study might be an important step forward to first clinical observational studies on the future discovery of gas plasma technology's potential in neurosurgery and neuro-oncology especially in putative adjuvant or combinatory GBM treatment settings.
Keywords: brain tumor; chemokines; cold physical plasma; cytokines; reactive oxygen species.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Patient-Derived Human Basal and Cutaneous Squamous Cell Carcinoma Tissues Display Apoptosis and Immunomodulation following Gas Plasma Exposure with a Certified Argon Jet.Int J Mol Sci. 2021 Oct 23;22(21):11446. doi: 10.3390/ijms222111446. Int J Mol Sci. 2021. PMID: 34768877 Free PMC article.
-
Gas Plasma-Treated Prostate Cancer Cells Augment Myeloid Cell Activity and Cytotoxicity.Antioxidants (Basel). 2020 Apr 16;9(4):323. doi: 10.3390/antiox9040323. Antioxidants (Basel). 2020. PMID: 32316245 Free PMC article.
-
Zerumbone Promotes Cytotoxicity in Human Malignant Glioblastoma Cells through Reactive Oxygen Species (ROS) Generation.Oxid Med Cell Longev. 2020 May 6;2020:3237983. doi: 10.1155/2020/3237983. eCollection 2020. Oxid Med Cell Longev. 2020. PMID: 32454937 Free PMC article.
-
Hyperbaric oxygen therapy as adjunctive strategy in treatment of glioblastoma multiforme.Med Gas Res. 2018 Apr 18;8(1):24-28. doi: 10.4103/2045-9912.229600. eCollection 2018 Jan-Mar. Med Gas Res. 2018. PMID: 29770193 Free PMC article. Review.
-
Cold Atmospheric Plasma as an Adjunct to Immunotherapy for Glioblastoma Multiforme.World Neurosurg. 2019 Oct;130:369-376. doi: 10.1016/j.wneu.2019.06.209. Epub 2019 Jul 5. World Neurosurg. 2019. PMID: 31284051 Review.
Cited by
-
Modulation of the Tumor-Associated Immuno-Environment by Non-Invasive Physical Plasma.Cancers (Basel). 2023 Feb 8;15(4):1073. doi: 10.3390/cancers15041073. Cancers (Basel). 2023. PMID: 36831415 Free PMC article. Review.
-
Comparative assessment of direct and indirect cold atmospheric plasma effects, based on helium and argon, on human glioblastoma: an in vitro and in vivo study.Sci Rep. 2024 Feb 13;14(1):3578. doi: 10.1038/s41598-024-54070-4. Sci Rep. 2024. PMID: 38347045 Free PMC article.
-
Medical gas plasma technology: Roadmap on cancer treatment and immunotherapy.Redox Biol. 2023 Sep;65:102798. doi: 10.1016/j.redox.2023.102798. Epub 2023 Jun 27. Redox Biol. 2023. PMID: 37556976 Free PMC article. Review.
References
-
- Bekeschus S., Schmidt A., Weltmann K.-D., von Woedtke T. The plasma jet kinpen—A powerful tool for wound healing. Clin. Plas. Med. 2016;4:19–28. doi: 10.1016/j.cpme.2016.01.001. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

