Modulation of DNA Damage Response by SAM and HD Domain Containing Deoxynucleoside Triphosphate Triphosphohydrolase (SAMHD1) Determines Prognosis and Treatment Efficacy in Different Solid Tumor Types
- PMID: 35158911
- PMCID: PMC8833711
- DOI: 10.3390/cancers14030641
Modulation of DNA Damage Response by SAM and HD Domain Containing Deoxynucleoside Triphosphate Triphosphohydrolase (SAMHD1) Determines Prognosis and Treatment Efficacy in Different Solid Tumor Types
Abstract
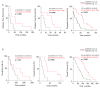
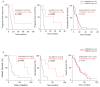
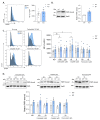
SAMHD1 is a deoxynucleotide triphosphate (dNTP) triphosphohydrolase with important roles in the control of cell proliferation and apoptosis, either through the regulation of intracellular dNTPs levels or the modulation of the DNA damage response. However, SAMHD1's role in cancer evolution is still unknown. We performed the first in-depth study of SAMHD1's role in advanced solid tumors, by analyzing samples of 128 patients treated with chemotherapy agents based on platinum derivatives and/or antimetabolites, developing novel in vitro knock-out models to explore the mechanisms driving SAMHD1 function in cancer. Low (or no) expression of SAMHD1 was associated with a positive prognosis in breast, ovarian, and non-small cell lung cancer (NSCLC) cancer patients. A predictive value was associated with low-SAMHD1 expression in NSCLC and ovarian patients treated with antimetabolites in combination with platinum derivatives. In vitro, SAMHD1 knock-out cells showed increased γ-H2AX and apoptosis, suggesting that SAMHD1 depletion induces DNA damage leading to cell death. In vitro treatment with platinum-derived drugs significantly enhanced γ-H2AX and apoptotic markers expression in knock-out cells, indicating a synergic effect of SAMHD1 depletion and platinum-based treatment. SAMHD1 expression represents a new strong prognostic and predictive biomarker in solid tumors and, thus, modulation of the SAMHD1 function may constitute a promising target for the improvement of cancer therapy.
Keywords: NSCLC; SAMHD1; breast cancer; ovarian cancer; solid tumors.
Conflict of interest statement
M.R. declares a consulting and advisory role for GSK, AstraZeneca, and MSD; and research funding from Pfizer, Clovis, GSK, AstraZeneca, and MSD. T.M. declares a consulting and advisory role for AstraZeneca, BMS, and MSD. L.L. declares a consulting or advisory role for Celgene and Sanofi and travel expenses from Sanofi, Merck, Roche, Amgen, and Ipsen. Ricard Mesia declares a consulting and advisory role for Merck, MSD, Roche, AstraZeneca, BEM, the speaker’s bureau for Merck, MSD, BMS, and Roche. M.M. declares a consulting and advisory role for Novartis, Pfizer, Pier Fabre, and Roche; research funding from Roche, Eisai, AstraZeneca, and travel expenses from Roche. The rest authors declare no potential conflict of interest.
Figures

Similar articles
-
Targeting SAMHD1: To overcome multiple anti-cancer drugs resistance in hematological malignancies.Genes Dis. 2022 Jun 26;10(3):891-900. doi: 10.1016/j.gendis.2022.06.001. eCollection 2023 May. Genes Dis. 2022. PMID: 37396510 Free PMC article. Review.
-
SAMHD1 expression modulates innate immune activation and correlates with ovarian cancer prognosis.Front Immunol. 2023 Feb 9;14:1112761. doi: 10.3389/fimmu.2023.1112761. eCollection 2023. Front Immunol. 2023. PMID: 36845138 Free PMC article.
-
SAMHD1 is a biomarker for cytarabine response and a therapeutic target in acute myeloid leukemia.Nat Med. 2017 Feb;23(2):250-255. doi: 10.1038/nm.4255. Epub 2016 Dec 19. Nat Med. 2017. PMID: 27991919
-
The deoxynucleotide triphosphohydrolase SAMHD1 is a major regulator of DNA precursor pools in mammalian cells.Proc Natl Acad Sci U S A. 2013 Aug 27;110(35):14272-7. doi: 10.1073/pnas.1312033110. Epub 2013 Jul 15. Proc Natl Acad Sci U S A. 2013. PMID: 23858451 Free PMC article.
-
SAMHD1 Functions and Human Diseases.Viruses. 2020 Mar 31;12(4):382. doi: 10.3390/v12040382. Viruses. 2020. PMID: 32244340 Free PMC article. Review.
Cited by
-
Targeting SAMHD1: To overcome multiple anti-cancer drugs resistance in hematological malignancies.Genes Dis. 2022 Jun 26;10(3):891-900. doi: 10.1016/j.gendis.2022.06.001. eCollection 2023 May. Genes Dis. 2022. PMID: 37396510 Free PMC article. Review.
-
SAMHD1 expression is a surrogate marker of immune infiltration and determines prognosis after neoadjuvant chemotherapy in early breast cancer.Cell Oncol (Dordr). 2024 Feb;47(1):189-208. doi: 10.1007/s13402-023-00862-1. Epub 2023 Sep 4. Cell Oncol (Dordr). 2024. PMID: 37667113 Free PMC article.
-
Unraveling the genetics of heat tolerance in chickpea landraces (Cicer arietinum L.) using genome-wide association studies.Front Plant Sci. 2024 Mar 25;15:1376381. doi: 10.3389/fpls.2024.1376381. eCollection 2024. Front Plant Sci. 2024. PMID: 38590753 Free PMC article.
-
Deciphering the role of SAMHD1 in endometrial cancer progression.Biol Direct. 2024 Oct 11;19(1):89. doi: 10.1186/s13062-024-00525-7. Biol Direct. 2024. PMID: 39394602 Free PMC article.
-
SAMHD1 expression modulates innate immune activation and correlates with ovarian cancer prognosis.Front Immunol. 2023 Feb 9;14:1112761. doi: 10.3389/fimmu.2023.1112761. eCollection 2023. Front Immunol. 2023. PMID: 36845138 Free PMC article.
References
-
- Knecht K.M., Buzovetsky O., Schneider C., Thomas D., Srikanth V., Kaderali L., Tofoleanu F., Reiss K., Ferreirós N., Geisslinger G., et al. The structural basis for cancer drug interactions with the catalytic and allosteric sites of SAMHD1. Proc. Natl. Acad. Sci. USA. 2018;115:E10022–E10031. doi: 10.1073/pnas.1805593115. - DOI - PMC - PubMed
-
- Arnold L.H., Groom H.C.T., Kunzelmann S., Schwefel D., Caswell S.J., Ordonez P., Mann M.C., Rueschenbaum S., Goldstone D.C., Pennell S., et al. Phospho-dependent Regulation of SAMHD1 Oligomerisation Couples Catalysis and Restriction. PLoS Pathog. 2015;11:e1005194. doi: 10.1371/journal.ppat.1005194. - DOI - PMC - PubMed
-
- Rice G.I., Bond J., Asipu A., Brunette R.L., Manfield I.W., Carr I.M., Fuller J.C., Jackson R.M., Lamb T., Briggs T.A., et al. Mutations involved in Aicardi-Goutières syndrome implicate SAMHD1 as regulator of the innate immune response. Nat. Genet. 2009;41:829–832. doi: 10.1038/ng.373. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

