Advancements, Challenges, and Future Directions in Tackling Glioblastoma Resistance to Small Kinase Inhibitors
- PMID: 35158868
- PMCID: PMC8833415
- DOI: 10.3390/cancers14030600
Advancements, Challenges, and Future Directions in Tackling Glioblastoma Resistance to Small Kinase Inhibitors
Abstract
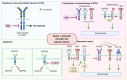
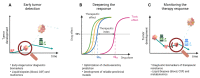
Despite clinical intervention, glioblastoma (GBM) remains the deadliest brain tumor in adults. Its incurability is partly related to the establishment of drug resistance, both to standard and novel treatments. In fact, even though small kinase inhibitors have changed the standard clinical practice for several solid cancers, in GBM, they did not fulfill this promise. Drug resistance is thought to arise from the heterogeneity of GBM, which leads the development of several different mechanisms. A better understanding of the evolution and characteristics of drug resistance is of utmost importance to improve the current clinical practice. Therefore, the development of clinically relevant preclinical in vitro models which allow careful dissection of these processes is crucial to gain insights that can be translated to improved therapeutic approaches. In this review, we first discuss the heterogeneity of GBM, which is reflected in the development of several resistance mechanisms. In particular, we address the potential role of drug resistance mechanisms in the failure of small kinase inhibitors in clinical trials. Finally, we discuss strategies to overcome therapy resistance, particularly focusing on the importance of developing in vitro models, and the possible approaches that could be applied to the clinic to manage drug resistance.
Keywords: cell culture models; drug resistance; glioblastoma; overcoming resistance; small kinase inhibitors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Opportunities and Challenges of Small Molecule Inhibitors in Glioblastoma Treatment: Lessons Learned from Clinical Trials.Cancers (Basel). 2024 Aug 29;16(17):3021. doi: 10.3390/cancers16173021. Cancers (Basel). 2024. PMID: 39272879 Free PMC article. Review.
-
Cell-based therapies for glioblastoma: Promising tools against tumor heterogeneity.Neuro Oncol. 2023 Sep 5;25(9):1551-1562. doi: 10.1093/neuonc/noad092. Neuro Oncol. 2023. PMID: 37179459 Free PMC article. Review.
-
Novel kinome profiling technology reveals drug treatment is patient and 2D/3D model dependent in glioblastoma.Front Oncol. 2022 Nov 3;12:1012236. doi: 10.3389/fonc.2022.1012236. eCollection 2022. Front Oncol. 2022. PMID: 36408180 Free PMC article.
-
Combinatorial approaches to effective therapy in glioblastoma (GBM): Current status and what the future holds.Int Rev Immunol. 2022;41(6):582-605. doi: 10.1080/08830185.2022.2101647. Epub 2022 Aug 8. Int Rev Immunol. 2022. PMID: 35938932 Review.
-
Major Challenges and Potential Microenvironment-Targeted Therapies in Glioblastoma.Int J Mol Sci. 2017 Dec 16;18(12):2732. doi: 10.3390/ijms18122732. Int J Mol Sci. 2017. PMID: 29258180 Free PMC article. Review.
Cited by
-
New Directions in the Therapy of Glioblastoma.Cancers (Basel). 2022 Oct 31;14(21):5377. doi: 10.3390/cancers14215377. Cancers (Basel). 2022. PMID: 36358795 Free PMC article. Review.
-
Current and future therapeutic strategies for high-grade gliomas leveraging the interplay between epigenetic regulators and kinase signaling networks.J Exp Clin Cancer Res. 2024 Jan 5;43(1):12. doi: 10.1186/s13046-023-02923-7. J Exp Clin Cancer Res. 2024. PMID: 38183103 Free PMC article. Review.
-
Glioblastoma mesenchymal subtype enhances antioxidant defence to reduce susceptibility to ferroptosis.Sci Rep. 2024 Sep 5;14(1):20770. doi: 10.1038/s41598-024-72024-8. Sci Rep. 2024. PMID: 39237744 Free PMC article.
-
Current Opportunities for Targeting Dysregulated Neurodevelopmental Signaling Pathways in Glioblastoma.Cells. 2022 Aug 15;11(16):2530. doi: 10.3390/cells11162530. Cells. 2022. PMID: 36010607 Free PMC article. Review.
-
Involvement of Cyclooxygenase-2 in Establishing an Immunosuppressive Microenvironment in Tumorspheres Derived from TMZ-Resistant Glioblastoma Cell Lines and Primary Cultures.Cells. 2024 Jan 30;13(3):258. doi: 10.3390/cells13030258. Cells. 2024. PMID: 38334650 Free PMC article.
References
-
- Gilbert M.R., Wang M., Aldape K.D., Stupp R., Hegi M.E., Jaeckle K.A., Armstrong T.S., Wefel J.S., Won M., Blumenthal D.T., et al. Dose-Dense Temozolomide for Newly Diagnosed Glioblastoma: A Randomized Phase III Clinical Trial. J. Clin. Oncol. 2013;31:4085–4091. doi: 10.1200/JCO.2013.49.6968. - DOI - PMC - PubMed
-
- Stupp R., Taillibert S., Kanner A.A., Read W., Steinberg D.M., Lhermitte B., Toms S., Idbaih A., Ahluwalia M.S., Fink K., et al. Effect of Tumor-Treating Fields Plus Maintenance Temozolomide vs Maintenance Temozolomide Alone on Survival in Patients with Glioblastoma: A Randomized Clinical Trial. JAMA. 2017;318:2306–2316. doi: 10.1001/jama.2017.18718. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

