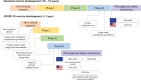
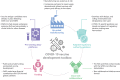
Clinical development and approval of COVID-19 vaccines
- PMID: 35157542
- PMCID: PMC8935460
- DOI: 10.1080/14760584.2022.2042257
Clinical development and approval of COVID-19 vaccines
Abstract
Introduction: The coronavirus 19 (COVID-19) pandemic triggered a simultaneous global demand for preventative vaccines, which quickly became a high priority among governments as well as academia and the pharmaceutical industry. Within less than a year after COVID-19 was declared a pandemic, vaccines had received emergency approvals and vaccination campaigns were initiated.
Areas covered: We discuss the several factors that led to the unprecedented, accelerated development and approval of COVID-19 vaccines, which includes optimization of processes by regulatory authorities, redesign of sequential development processes, learnings from previous pandemics, and prior development of novel vaccine platforms.
Expert opinion: Despite unanticipated and complex challenges presented by real-time vaccine development in the context of the evolving COVID-19 pandemic and subsequent ever-changing landscape of public health measures and recommendations, important milestones were reached within extraordinarily short periods and, following roll-out to billions worldwide, the approved vaccines have proven to be well tolerated and effective. Whilst this is an exceptional feat and an example of what can be achieved with collaboration and innovation, there are lessons that can still be learned, including the need for further harmonization between regulatory authorities, modes to react to the pandemic's ever-evolving challenges, and ensuring equitable vaccine access among low-income countries.
Keywords: COVID-19; pandemic; public health; regulatory processes; vaccine development.
Figures
Similar articles
-
COVID-19 and vaccination: myths vs science.Expert Rev Vaccines. 2022 Nov;21(11):1603-1620. doi: 10.1080/14760584.2022.2114900. Epub 2022 Sep 1. Expert Rev Vaccines. 2022. PMID: 35980281
-
The race for a COVID-19 vaccine: where are we up to?Expert Rev Vaccines. 2022 Mar;21(3):355-376. doi: 10.1080/14760584.2022.2021074. Epub 2021 Dec 28. Expert Rev Vaccines. 2022. PMID: 34937492
-
International Collaboration to Ensure Equitable Access to Vaccines for COVID-19: The ACT-Accelerator and the COVAX Facility.Milbank Q. 2021 Jun;99(2):426-449. doi: 10.1111/1468-0009.12503. Epub 2021 Mar 2. Milbank Q. 2021. PMID: 33650737 Free PMC article.
-
How to accelerate the supply of vaccines to all populations worldwide? Part I: Initial industry lessons learned and practical overarching proposals leveraging the COVID-19 situation.Vaccine. 2022 Feb 23;40(9):1215-1222. doi: 10.1016/j.vaccine.2021.11.098. Vaccine. 2022. PMID: 35180993 Free PMC article. Review.
-
Factors, enablers and challenges for COVID-19 vaccine development.BMJ Glob Health. 2023 Jun;8(6):e011879. doi: 10.1136/bmjgh-2023-011879. BMJ Glob Health. 2023. PMID: 37277195 Free PMC article. Review.
Cited by
-
COVID-19 Vaccines over Three Years after the Outbreak of the COVID-19 Epidemic.Viruses. 2023 Aug 23;15(9):1786. doi: 10.3390/v15091786. Viruses. 2023. PMID: 37766194 Free PMC article. Review.
-
Engineering a SARS-CoV-2 Vaccine Targeting the Receptor-Binding Domain Cryptic-Face via Immunofocusing.ACS Cent Sci. 2024 Sep 17;10(10):1871-1884. doi: 10.1021/acscentsci.4c00722. eCollection 2024 Oct 23. ACS Cent Sci. 2024. PMID: 39463836 Free PMC article.
-
Investing in a global pooled-funding mechanism for late-stage clinical trials of poverty-related and neglected diseases: an economic evaluation.BMJ Glob Health. 2023 May;8(5):e011842. doi: 10.1136/bmjgh-2023-011842. BMJ Glob Health. 2023. PMID: 37247874 Free PMC article.
-
From the internet to the COVID-19 pandemic: how technological advances and a tumultuous world have changed scientific publishing and meetings.J Nephrol. 2023 Nov;36(8):2165-2167. doi: 10.1007/s40620-023-01740-7. J Nephrol. 2023. PMID: 37498469 No abstract available.
-
Pulmonary embolism after SARS-CoV-2 vaccination.Vaccine X. 2024 Oct 15;21:100571. doi: 10.1016/j.jvacx.2024.100571. eCollection 2024 Dec. Vaccine X. 2024. PMID: 39474208 Free PMC article.
References
-
- World Health Organization . Coronavirus Disease (COVID-19). Press Conference, 28 February 2020 [Internet]. [cited 2022 Feb 10]. Available from: https://www.who.int/docs/default-source/coronaviruse/transcripts/who-aud...
-
- World Health Organization . WHO director-general’s opening remarks at the media briefing on COVID-19 - 11 March 2020 [Internet]. [cited 2022 Feb 10]. Available from: https://www.who.int/director-general/speeches/detail/who-director-genera...
-
- Le TT, Cramer JP, Chen R, et al. Evolution of the COVID-19 vaccine development landscape. Nat Rev Drug Discov. 2020;19:667–668. - PubMed
-
- US Food and Drug Administration . Emergency Use Authorization (EUA) for an unapproved product review memorandum. Application 27034 [Internet]. [cited 2022 Feb 10]. Available from: https://www.fda.gov/media/144416/download
-
- US Food and Drug Administration . Emergency Use Authorization (EUA) for an unapproved product review memorandum. Application 27073 [Internet]. [cited 2022 Feb 10]. Available from: https://fda.report/media/144673/Moderna+COVID-19+Vaccine+review+memo.pdf
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical