The utility of remdesivir in SARS-CoV-2: A single tertiary care center experience from a developing country
- PMID: 35156078
- PMCID: PMC8823973
- DOI: 10.1016/j.rcsop.2022.100107
The utility of remdesivir in SARS-CoV-2: A single tertiary care center experience from a developing country
Abstract
Background: Remdesivir is a monophosphoramidate prodrug of an adenosine analog, and it has a broad-spectrum antiviral activity against paramyxoviruses, flaviviruses, and coronaviruses. Remdesivir is associated with decreased hospital stay and improved outcomes in coronavirus- disease 2019 (COVID-19).
Methodology: Of 846 suspected COVID-19 patients admitted to the hospital, 612 SARS-CoV-2 nasopharyngeal RT-PCR positive patients were evaluated for enrollment in this prospective cohort study. 159 RT-PCR positive patients were given remdesivir. Their clinical, biochemical parameters, hospital stay, and outcomes related to morbidity and mortality were followed.
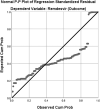
Results: Out of the 159 patients, 141 recovered after remdesivir use. The Chi-square test for independence examined the relation between the day of the first dose, dose of remdesivir, and clinical outcome. The standardized case fatality ratio (CFR) in the 453 hospitalized patients who did not receive remdesivir was 32.89% (N = 149) as compared to 11.32% (N = 18) in the patients who received remdesivir. These findings are in keeping with the therapeutic value of remdesivir in symptomatic SARS-CoV-2 infection of varying severity.
Conclusion: The use of remdesivir is associated with a decrease in the severity of the SARS-CoV-2 infection. Its use is also associated with a decreased length of hospital stay and lower mortality than the patients who did not receive remdesivir.
Keywords: COVID-19; Drug safety; Infection; Pakistan; Remdesivir; Treatment.
© 2022 The Authors.
Conflict of interest statement
No conflict of interest among the authors.
Figures
Similar articles
-
Evaluating the Clinical Outcomes of Remdesivir Among Patients Admitted With COVID-19 in a Tertiary Care Hospital.Cureus. 2021 Nov 11;13(11):e19487. doi: 10.7759/cureus.19487. eCollection 2021 Nov. Cureus. 2021. Retraction in: Cureus. 2022 Mar 17;14(3):r44. doi: 10.7759/cureus.r44. PMID: 34912628 Free PMC article. Retracted.
-
Safety and Efficacy of Imatinib for Hospitalized Adults with COVID-19: A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Oct 28;21(1):897. doi: 10.1186/s13063-020-04819-9. Trials. 2020. PMID: 33115543 Free PMC article.
-
Qualitative Subgenomic RNA to Monitor the Response to Remdesivir in Hospitalized Patients With Coronavirus Disease 2019: Impact on the Length of Hospital Stay and Mortality.Clin Infect Dis. 2023 Jan 6;76(1):32-38. doi: 10.1093/cid/ciac760. Clin Infect Dis. 2023. PMID: 36097825 Free PMC article.
-
Remdesivir in treating hospitalized patients with COVID-19: A renewed review of clinical trials.Front Pharmacol. 2022 Sep 8;13:971890. doi: 10.3389/fphar.2022.971890. eCollection 2022. Front Pharmacol. 2022. PMID: 36160434 Free PMC article. Review.
-
Current knowledge about the antivirals remdesivir (GS-5734) and GS-441524 as therapeutic options for coronaviruses.One Health. 2020 Mar 27;9:100128. doi: 10.1016/j.onehlt.2020.100128. eCollection 2020 Jun. One Health. 2020. PMID: 32258351 Free PMC article. Review.
Cited by
-
Efficacy of Remdesivir on Clinical Outcomes in COVID-19 Patients: A Study in a Tertiary Care Hospital in Pakistan.J Community Hosp Intern Med Perspect. 2024 May 7;14(3):25-31. doi: 10.55729/2000-9666.1333. eCollection 2024. J Community Hosp Intern Med Perspect. 2024. PMID: 39036580 Free PMC article.
References
-
- Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA - J Am Med Assoc. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. - DOI - PubMed
-
- Muhammad Alfareed Zafar S., Junaid Tahir M., Malik M., Irfan Malik M., Kamal Akhtar F., Ghazala R. Awareness, anxiety, and depression in healthcare professionals, medical students, and general population of Pakistan during COVID-19 pandemic: a cross sectional online survey. Med. J. Islam Repub. Iran. 2020;34 doi: 10.34171/mjiri.34.131. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous