Human pancreatic tumour organoid-derived factors enhance myogenic differentiation
- PMID: 35146962
- PMCID: PMC8977981
- DOI: 10.1002/jcsm.12917
Human pancreatic tumour organoid-derived factors enhance myogenic differentiation
Abstract
Background: Most patients with pancreatic cancer develop cachexia, which is characterized by progressive muscle loss. The mechanisms underlying muscle loss in cancer cachexia remain elusive. Pancreatic tumour organoids are 3D cell culture models that retain key characteristics of the parent tumour. We aimed to investigate the effect of pancreatic tumour organoid-derived factors on processes that determine skeletal muscle mass, including the regulation of muscle protein turnover and myogenesis.
Methods: Conditioned medium (CM) was collected from human pancreatic cancer cell lines (PK-45H, PANC-1, PK-1, and KLM-1), pancreatic tumour organoid cultures from a severely cachectic (PANCO-9a) and a non-cachectic patient (PANCO-12a), and a normal pancreas organoid culture. Differentiating C2C12 myoblasts and mature C2C12 myotubes were exposed to CM for 24 h or maintained in control medium. In myotubes, NF-kB activation was monitored using a NF-κB luciferase reporter construct, and mRNA expression of E3-ubiquitin ligases and REDD1 was analysed by RT-qPCR. C2C12 myoblast proliferation and differentiation were monitored by live cell imaging and myogenic markers and myosin heavy chain (MyHC) isoforms were assessed by RT-qPCR.
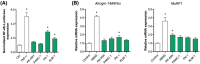
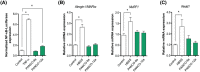
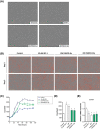
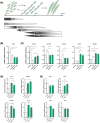
Results: Whereas CM from PK-1 and KLM-1 cells significantly induced NF-κB activation in C2C12 myotubes (PK-1: 3.1-fold, P < 0.001; KLM-1: 2.1-fold, P = 0.01), Atrogin-1/MAFbx and MuRF1 mRNA were only minimally and inconsistently upregulated by the CM of pancreatic cancer cell lines. Similarly, E3-ubiquitin ligases and REDD1 mRNA expression in myotubes were not altered by exposure to pancreatic tumour organoid CM. Compared with the control condition, CM from both PANCO-9a and PANCO-12a tumour organoids increased proliferation of myoblasts, which was accompanied by significant downregulation of the satellite cell marker paired-box 7 (PAX7) (PANCO-9a: -2.1-fold, P < 0.001; PANCO-12a: -2.0-fold, P < 0.001) and myogenic factor 5 (MYF5) (PANCO-9a: -2.1-fold, P < 0.001; PANCO-12a: -1.8-fold, P < 0.001) after 48 h of differentiation. Live cell imaging revealed accelerated alignment and fusion of myoblasts exposed to CM from PANCO-9a and PANCO-12a, which was in line with significantly increased Myomaker mRNA expression levels (PANCO-9a: 2.4-fold, P = 0.001; PANCO-12a: 2.2-fold, P = 0.004). These morphological and transcriptional alterations were accompanied by increased expression of muscle differentiation markers such as MyHC-IIB (PANCO-9a: 2.5-fold, P = 0.04; PANCO-12a: 3.1-fold, P = 0.006). Although the impact of organoid CM on myogenesis was not associated with the cachexia phenotype of the donor patients, it was specific for tumour organoids, as CM of control pancreas organoids did not modulate myogenic fusion.
Conclusions: These data show that pancreatic tumour organoid-derived factors alter the kinetics of myogenesis, which may eventually contribute to impaired muscle mass maintenance in cancer cachexia.
Keywords: Cachexia; E3 ubiquitin ligases; Myogenesis; Organoids; Skeletal muscle atrophy.
© 2022 The Authors. Journal of Cachexia, Sarcopenia and Muscle published by John Wiley & Sons Ltd on behalf of Society on Sarcopenia, Cachexia and Wasting Disorders.
Conflict of interest statement
The authors have nothing to disclose.
Figures
Similar articles
-
Generation and initial characterization of novel tumour organoid models to study human pancreatic cancer-induced cachexia.J Cachexia Sarcopenia Muscle. 2020 Dec;11(6):1509-1524. doi: 10.1002/jcsm.12627. Epub 2020 Oct 13. J Cachexia Sarcopenia Muscle. 2020. PMID: 33047901 Free PMC article.
-
Extracellular vesicles derived from tumour cells as a trigger of energy crisis in the skeletal muscle.J Cachexia Sarcopenia Muscle. 2022 Feb;13(1):481-494. doi: 10.1002/jcsm.12844. Epub 2021 Dec 20. J Cachexia Sarcopenia Muscle. 2022. PMID: 34931471 Free PMC article.
-
Cryptotanshinone prevents muscle wasting in CT26-induced cancer cachexia through inhibiting STAT3 signaling pathway.J Ethnopharmacol. 2020 Oct 5;260:113066. doi: 10.1016/j.jep.2020.113066. Epub 2020 Jun 4. J Ethnopharmacol. 2020. PMID: 32505837
-
PI3 kinase regulation of skeletal muscle hypertrophy and atrophy.Curr Top Microbiol Immunol. 2010;346:267-78. doi: 10.1007/82_2010_78. Curr Top Microbiol Immunol. 2010. PMID: 20593312 Review.
-
Signaling pathways perturbing muscle mass.Curr Opin Clin Nutr Metab Care. 2010 May;13(3):225-9. doi: 10.1097/mco.0b013e32833862df. Curr Opin Clin Nutr Metab Care. 2010. PMID: 20397318 Review.
Cited by
-
DUSP1 promotes muscle atrophy by inhibiting myocyte differentiation in cachectic patients.Front Oncol. 2022 Nov 1;12:1040112. doi: 10.3389/fonc.2022.1040112. eCollection 2022. Front Oncol. 2022. PMID: 36387242 Free PMC article.
-
Extracellular Vesicles Inhibit the Response of Pancreatic Ductal Adenocarcinoma Cells to Gemcitabine and TRAIL Treatment.Int J Mol Sci. 2022 Jul 15;23(14):7810. doi: 10.3390/ijms23147810. Int J Mol Sci. 2022. PMID: 35887158 Free PMC article.
-
Hesperetin but not ellagic acid increases myosin heavy chain expression and cell fusion in C2C12 myoblasts in the presence of oxidative stress.Front Nutr. 2024 Sep 2;11:1377071. doi: 10.3389/fnut.2024.1377071. eCollection 2024. Front Nutr. 2024. PMID: 39285862 Free PMC article.
-
Pancreatic Tumor Organoid-Derived Factors from Cachectic Patients Disrupt Contractile Smooth Muscle Cells.Cancers (Basel). 2024 Jan 26;16(3):542. doi: 10.3390/cancers16030542. Cancers (Basel). 2024. PMID: 38339292 Free PMC article.
-
Extracellular vesicles in cancer cachexia: deciphering pathogenic roles and exploring therapeutic horizons.J Transl Med. 2024 May 27;22(1):506. doi: 10.1186/s12967-024-05266-9. J Transl Med. 2024. PMID: 38802952 Free PMC article. Review.
References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. - PubMed
-
- Kleeff J, Korc M, Apte M, La Vecchia C, Johnson CD, Biankin AV, et al. Pancreatic cancer. Nat Rev Dis Primers 2016;2:16022. - PubMed
-
- Baracos VE, Martin L, Korc M, Guttridge DC, Fearon KCH. Cancer‐associated cachexia. Nat Rev Dis Primers 2018;4:17105. - PubMed
-
- Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011;12:489–495. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

