Temporary Shutdown of ERK1/2 Phosphorylation Is Associated With Activation of Adaptive Immune Cell Responses and Disease Progression During Leishmania amazonensis Infection in BALB/c Mice
- PMID: 35145518
- PMCID: PMC8821891
- DOI: 10.3389/fimmu.2022.762080
Temporary Shutdown of ERK1/2 Phosphorylation Is Associated With Activation of Adaptive Immune Cell Responses and Disease Progression During Leishmania amazonensis Infection in BALB/c Mice
Abstract
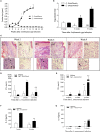
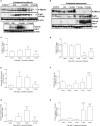
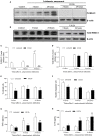
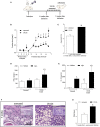
Leishmania spp. infection outcomes are dependent on both host and parasite factors. Manipulation of host signaling pathways involved in the generation of immune responses is thought to be one of the most common mechanisms used by parasites for persistence within the host. Considering the diversity of pathologies caused by different Leishmania spp., it is plausible that significant differences may exist in the mechanisms of host cell manipulation by each parasite species, which may have implications when developing new vaccine or treatment strategies. Here we show that in L. braziliensis-infection in BALB/c mice, a model of resistance, activation of ERK1/2 coincides with the peak of inflammatory responses and resolution of tissue parasitism. In contrast, in the susceptibility model of L. amazonensis-infection, an early silent phase of infection is observed, detected solely by quantification of parasite loads. At this early stage, only basal levels of P-ERK1/2 are observed. Later, after a brief shutdown of ERK1/2 phosphorylation, disease progression is observed and is associated with increased inflammation, lesion size and tissue parasitism. Moreover, the short-term down-regulation of ERK1/2 activation affected significantly downstream inflammatory pathways and adaptive T cell responses. Administration of U0126, a MEK/ERK inhibitor, confirmed this phenomenon, since bigger lesions and higher parasite loads were seen in infected mice that received U0126. To investigate how kinetics of ERK1/2 activation could affect the disease progression, U0126 was administered to L. amazonensis-infected animals earlier than the P-ERK1/2 switch off time-point. This intervention resulted in anticipation of the same effects on inflammatory responses and susceptibility phenotype seen in the natural course of infection. Additionally, in vitro inhibition of ERK1/2 affected the phagocytosis of L. amazonensis by BMDMs. Collectively, our findings reveal distinct temporal patterns of activation of inflammatory responses in L. braziliensis and L. amazonensis in the same animal background and a pivotal role for a brief and specific shutdown of ERK1/2 activation at late stages of L. amazonensis infection. Since activation of inflammatory responses is a crucial aspect for the control of infectious processes, these findings may be important for the search of new and specific strategies of vaccines and treatment for tegumentary leishmaniasis.
Keywords: L. amazonensis; L. braziliensis; inflammation; kinetics of ERK1/2 activation; leishmaniasis infection; treatment; vaccine.
Copyright © 2022 Oliveira, Souza-Testasicca, Ricotta, Vago, dos Santos, Crepaldi, Lima, Queiroz-Junior, Sousa and Fernandes.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Impact of reactive oxygen species (ROS) on the control of parasite loads and inflammation in Leishmania amazonensis infection.Parasit Vectors. 2016 Apr 7;9:193. doi: 10.1186/s13071-016-1472-y. Parasit Vectors. 2016. PMID: 27056545 Free PMC article.
-
IL-32γ promotes the healing of murine cutaneous lesions caused by Leishmania braziliensis infection in contrast to Leishmania amazonensis.Parasit Vectors. 2017 Jul 14;10(1):336. doi: 10.1186/s13071-017-2268-4. Parasit Vectors. 2017. PMID: 28709468 Free PMC article.
-
A vaccine composed of a hypothetical protein and the eukaryotic initiation factor 5a from Leishmania braziliensis cross-protection against Leishmania amazonensis infection.Immunobiology. 2017 Feb;222(2):251-260. doi: 10.1016/j.imbio.2016.09.015. Epub 2016 Sep 28. Immunobiology. 2017. PMID: 27693018
-
The Paradox of a Phagosomal Lifestyle: How Innate Host Cell-Leishmania amazonensis Interactions Lead to a Progressive Chronic Disease.Front Immunol. 2021 Sep 7;12:728848. doi: 10.3389/fimmu.2021.728848. eCollection 2021. Front Immunol. 2021. PMID: 34557194 Free PMC article. Review.
-
Leishmania species-dependent functional duality of toll-like receptor 2.IUBMB Life. 2019 Nov;71(11):1685-1700. doi: 10.1002/iub.2129. Epub 2019 Jul 22. IUBMB Life. 2019. PMID: 31329370 Review.
Cited by
-
MicroRNA-194 regulates parasitic load and IL-1β-dependent nitric oxide production in the peripheral blood mononuclear cells of dogs with leishmaniasis.PLoS Negl Trop Dis. 2024 Jan 19;18(1):e0011789. doi: 10.1371/journal.pntd.0011789. eCollection 2024 Jan. PLoS Negl Trop Dis. 2024. PMID: 38241360 Free PMC article.
-
The state of the art of extracellular vesicle research in protozoan infection.Front Genet. 2022 Aug 12;13:941561. doi: 10.3389/fgene.2022.941561. eCollection 2022. Front Genet. 2022. PMID: 36035188 Free PMC article. Review.
References
-
- Carvalho AK, Silveira FT, Passero LF, Gomes CM, Corbett CE, Laurenti MD. Leishmania (V.) Braziliensis and L. (L.) Amazonensis Promote Differential Expression of Dendritic Cells and Cellular Immune Response in Murine Model. Parasite Immunol (2012) 34(8-9):395–403. doi: 10.1111/j.1365-3024.2012.01370.x - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

