Optimizing environmental safety and cell-killing potential of oncolytic Newcastle Disease virus with modifications of the V, F and HN genes
- PMID: 35139115
- PMCID: PMC8827430
- DOI: 10.1371/journal.pone.0263707
Optimizing environmental safety and cell-killing potential of oncolytic Newcastle Disease virus with modifications of the V, F and HN genes
Abstract
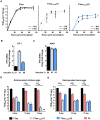
Newcastle Disease Virus (NDV) is an avian RNA virus, which was shown to be effective and safe for use in oncolytic viral therapy for several tumour malignancies. The presence of a multi basic cleavage site (MBCS) in the fusion protein improved its oncolytic efficacy in vitro and in vivo. However, NDV with a MBCS can be virulent in poultry. We aimed to develop an NDV with a MBCS but with reduced virulence for poultry while remaining effective in killing human tumour cells. To this end, the open reading frame of the V protein, an avian specific type I interferon antagonist, was disrupted by introducing multiple mutations. NDV with a mutated V gene was attenuated in avian cells and chicken and duck eggs. Although this virus still killed tumour cells, the efficacy was reduced compared to the virulent NDV. Introduction of various mutations in the fusion (F) and hemagglutinin-neuraminidase (HN) genes slightly improved this efficacy. Taken together, these data demonstrated that NDV with a MBCS but with abrogation of the V protein ORF and mutations in the F and HN genes can be safe for evaluation in oncolytic viral therapy.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Roles of the fusion and hemagglutinin-neuraminidase proteins in replication, tropism, and pathogenicity of avian paramyxoviruses.J Virol. 2011 Sep;85(17):8582-96. doi: 10.1128/JVI.00652-11. Epub 2011 Jun 15. J Virol. 2011. PMID: 21680512 Free PMC article.
-
Genetic Modification of Oncolytic Newcastle Disease Virus for Cancer Therapy.J Virol. 2016 May 12;90(11):5343-5352. doi: 10.1128/JVI.00136-16. Print 2016 Jun 1. J Virol. 2016. PMID: 27009956 Free PMC article.
-
Newcastle Disease Virus Establishes Persistent Infection in Tumor Cells In Vitro: Contribution of the Cleavage Site of Fusion Protein and Second Sialic Acid Binding Site of Hemagglutinin-Neuraminidase.J Virol. 2017 Jul 27;91(16):e00770-17. doi: 10.1128/JVI.00770-17. Print 2017 Aug 15. J Virol. 2017. PMID: 28592535 Free PMC article.
-
Newcastle disease virus as an oncolytic agent.Indian J Med Res. 2009 Nov;130(5):507-13. Indian J Med Res. 2009. PMID: 20090097 Review.
-
[Progress in using Newcastle disease virus for tumor therapy: a review].Sheng Wu Gong Cheng Xue Bao. 2010 Aug;26(8):1031-6. Sheng Wu Gong Cheng Xue Bao. 2010. PMID: 21090105 Review. Chinese.
Cited by
-
The V protein in oncolytic Newcastle disease virus promotes HepG2 hepatoma cell proliferation at the single-cell level.BMC Cancer. 2023 Apr 17;23(1):346. doi: 10.1186/s12885-023-10815-4. BMC Cancer. 2023. PMID: 37069523 Free PMC article.
-
Enhanced Oncolytic Potential of Engineered Newcastle Disease Virus Lasota Strain through Modification of Its F Protein Cleavage Site.Microorganisms. 2024 Oct 8;12(10):2029. doi: 10.3390/microorganisms12102029. Microorganisms. 2024. PMID: 39458338 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

