SARM1 knockout does not rescue neuromuscular phenotypes in a Charcot-Marie-Tooth disease Type 1A mouse model
- PMID: 35137510
- PMCID: PMC8940700
- DOI: 10.1111/jns.12483
SARM1 knockout does not rescue neuromuscular phenotypes in a Charcot-Marie-Tooth disease Type 1A mouse model
Abstract
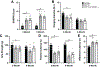
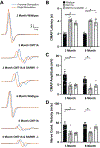
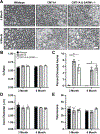
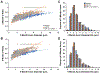
Charcot-Marie-Tooth disease Type 1A (CMT1A) is caused by duplication of the PMP22 gene and is the most common inherited peripheral neuropathy. Although CMT1A is a dysmyelinating peripheral neuropathy, secondary axon degeneration has been suggested to drive functional deficits in patients. Given that SARM1 knockout is a potent inhibitor of the programmed axon degeneration pathway, we asked whether SARM1 knockout rescues neuromuscular phenotypes in CMT1A model (C3-PMP) mice. CMT1A mice were bred with SARM1 knockout mice to generate CMT1A/SARM1-/- mice. A series of behavioral assays were employed to evaluate motor and sensorimotor function. Electrophysiological and histological studies of the tibial branch of the sciatic nerve were performed. Additionally, gastrocnemius and soleus muscle morphology were evaluated histologically. Although clear behavioral and electrophysiological deficits were observed in CMT1A model mice, genetic deletion of SARM1 conferred no significant improvement. Nerve morphometry revealed predominantly myelin deficits in CMT1A model mice and SARM1 knockout yielded no improvement in all nerve morphometry measures. Similarly, muscle morphometry deficits in CMT1A model mice were not improved by SARM1 knockout. Our findings demonstrate that programmed axon degeneration pathway inhibition does not provide therapeutic benefit in C3-PMP CMT1A model mice. Our results indicate that the clinical phenotypes observed in CMT1A mice are likely caused primarily by prolonged dysmyelination, motivate further investigation into mechanisms of dysmyelination in these mice and necessitate the development of improved CMT1A rodent models that recapitulate the secondary axon degeneration observed in patients.
Keywords: CMT1A; SARM1; behavior; electrophysiology; histology.
© 2022 Peripheral Nerve Society.
Figures

Similar articles
-
An essential role of MAG in mediating axon-myelin attachment in Charcot-Marie-Tooth 1A disease.Neurobiol Dis. 2013 Jan;49:221-31. doi: 10.1016/j.nbd.2012.08.009. Epub 2012 Aug 25. Neurobiol Dis. 2013. PMID: 22940629 Free PMC article.
-
Charcot-Marie-Tooth disease and related inherited neuropathies.Medicine (Baltimore). 1996 Sep;75(5):233-50. doi: 10.1097/00005792-199609000-00001. Medicine (Baltimore). 1996. PMID: 8862346 Review.
-
Synergistic PXT3003 therapy uncouples neuromuscular function from dysmyelination in male Charcot-Marie-Tooth disease type 1A (CMT1A) rats.J Neurosci Res. 2020 Oct;98(10):1933-1952. doi: 10.1002/jnr.24679. Epub 2020 Jun 26. J Neurosci Res. 2020. PMID: 32588471
-
Testing SIPA1L2 as a modifier of CMT1A using mouse models.J Neuropathol Exp Neurol. 2024 Apr 19;83(5):318-330. doi: 10.1093/jnen/nlae020. J Neuropathol Exp Neurol. 2024. PMID: 38472136 Free PMC article.
-
Targeting the programmed axon degeneration pathway as a potential therapeutic for Charcot-Marie-Tooth disease.Brain Res. 2020 Jan 15;1727:146539. doi: 10.1016/j.brainres.2019.146539. Epub 2019 Nov 2. Brain Res. 2020. PMID: 31689415 Free PMC article. Review.
Cited by
-
hESC- and hiPSC-derived Schwann cells are molecularly comparable and functionally equivalent.iScience. 2024 Apr 30;27(6):109855. doi: 10.1016/j.isci.2024.109855. eCollection 2024 Jun 21. iScience. 2024. PMID: 38770143 Free PMC article.
-
Application Research of Tooth Arrangement Based on Rotation Matrix Calculation and Resistance Detection in Oral.Comput Intell Neurosci. 2022 May 20;2022:4675181. doi: 10.1155/2022/4675181. eCollection 2022. Comput Intell Neurosci. 2022. PMID: 35634084 Free PMC article.
-
Axolemmal nanoruptures arising from paranodal membrane injury induce secondary axon degeneration in murine Guillain-Barré syndrome.J Peripher Nerv Syst. 2023 Mar;28(1):17-31. doi: 10.1111/jns.12532. Epub 2023 Feb 12. J Peripher Nerv Syst. 2023. PMID: 36710500 Free PMC article.
References
-
- Brennan KM, Bai Y, Shy ME. Demyelinating CMT--what’s known, what’s new and what’s in store? Neurosci Lett. 2015; 59614–26. - PubMed
-
- Krajewski KM, Lewis RA, Fuerst DR, Turansky C, Hinderer SR, Garbern J, Kamholz J, Shy ME. Neurological dysfunction and axonal degeneration in Charcot-Marie-Tooth disease type 1A. Brain : a journal of neurology. 2000; 123 (Pt 7)1516–1527. - PubMed
-
- Wang MS, Davis AA, Culver DG, Glass JD. WldS mice are resistant to paclitaxel (taxol) neuropathy. Annals of neurology. 2002; 52(4): 442–447. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

