MERS-CoV nsp1 impairs the cellular metabolic processes by selectively downregulating mRNAs in a novel granules
- PMID: 35129074
- PMCID: PMC8824216
- DOI: 10.1080/21505594.2022.2032928
MERS-CoV nsp1 impairs the cellular metabolic processes by selectively downregulating mRNAs in a novel granules
Abstract
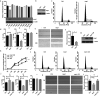
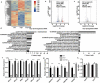
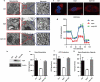
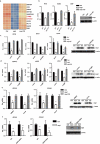
MERS-CoV infection can damage the cellular metabolic processes, but the underlying mechanisms are largely unknown. Through screening, we found non-structural protein 1 (nsp1) of MERS-CoV could inhibit cell viability, cell cycle, and cell migration through its endonuclease activity. Transcriptome sequencing revealed that MERS-CoV nsp1 specifically downregulated the mRNAs of ribosomal protein genes, oxidative phosphorylation protein genes, and antigen presentation genes, but upregulated the mRNAs of transcriptional regulatory genes. Further analysis shown nsp1 existed in a novel ribonucleosome complex formed via liquid-liquid phase separation, which did not co-localize with mitochondria, lysosomes, P-bodies, or stress granules. Interestingly, the nsp1-located granules specifically contained mRNAs of ribosomal protein genes and oxidative phosphorylation genes, which may explain why MERS-CoV nsp1 selectively degraded these mRNAs in cells. Finally, MERS-CoV nsp1 transgenic mice showed significant loss of body weight and an increased sensitivity to poly(I:C)-induced inflammatory death. These findings demonstrate a new mechanism by which MERS-CoV impairs cell viability, which serves as a potential novel target for preventing MERS-CoV infection-induced pathological damage.Abbreviations: (Middle East respiratory syndrome coronavirus (MERS-CoV), Actinomycin D (Act D), liquid-liquid phase separation (LLPS), stress granules (SGs), Mass spectrometry (IP-MS), RNA Binding Protein Immunoprecipitation (RIP)).
Keywords: LLPS; MERS-CoV; nsp1; oxidative phosphorylation; ribosome.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures

Similar articles
-
MERS-CoV nsp1 regulates autophagic flux via mTOR signalling and dysfunctional lysosomes.Emerg Microbes Infect. 2022 Dec;11(1):2529-2543. doi: 10.1080/22221751.2022.2128434. Emerg Microbes Infect. 2022. PMID: 36153658 Free PMC article.
-
Middle East Respiratory Syndrome Coronavirus nsp1 Inhibits Host Gene Expression by Selectively Targeting mRNAs Transcribed in the Nucleus while Sparing mRNAs of Cytoplasmic Origin.J Virol. 2015 Nov;89(21):10970-81. doi: 10.1128/JVI.01352-15. Epub 2015 Aug 26. J Virol. 2015. PMID: 26311885 Free PMC article.
-
The Endonucleolytic RNA Cleavage Function of nsp1 of Middle East Respiratory Syndrome Coronavirus Promotes the Production of Infectious Virus Particles in Specific Human Cell Lines.J Virol. 2018 Oct 12;92(21):e01157-18. doi: 10.1128/JVI.01157-18. Print 2018 Nov 1. J Virol. 2018. PMID: 30111568 Free PMC article.
-
Mechanisms of Coronavirus Nsp1-Mediated Control of Host and Viral Gene Expression.Cells. 2021 Feb 2;10(2):300. doi: 10.3390/cells10020300. Cells. 2021. PMID: 33540583 Free PMC article. Review.
-
Modulation of the immune response by Middle East respiratory syndrome coronavirus.J Cell Physiol. 2019 Mar;234(3):2143-2151. doi: 10.1002/jcp.27155. Epub 2018 Aug 26. J Cell Physiol. 2019. PMID: 30146782 Free PMC article. Review.
Cited by
-
Nsp1 proteins of human coronaviruses HCoV-OC43 and SARS-CoV2 inhibit stress granule formation.PLoS Pathog. 2022 Dec 19;18(12):e1011041. doi: 10.1371/journal.ppat.1011041. eCollection 2022 Dec. PLoS Pathog. 2022. PMID: 36534661 Free PMC article.
-
An Evolutionarily Conserved Strategy for Ribosome Binding and Host Translation Inhibition by β-coronavirus Non-structural Protein 1.J Mol Biol. 2023 Oct 15;435(20):168259. doi: 10.1016/j.jmb.2023.168259. Epub 2023 Sep 1. J Mol Biol. 2023. PMID: 37660941 Free PMC article.
-
MERS-CoV nsp1 regulates autophagic flux via mTOR signalling and dysfunctional lysosomes.Emerg Microbes Infect. 2022 Dec;11(1):2529-2543. doi: 10.1080/22221751.2022.2128434. Emerg Microbes Infect. 2022. PMID: 36153658 Free PMC article.
-
PSMD12 promotes non-small cell lung cancer progression through activating the Nrf2/TrxR1 pathway.Genes Genomics. 2024 Mar;46(3):263-277. doi: 10.1007/s13258-023-01484-5. Epub 2024 Jan 19. Genes Genomics. 2024. PMID: 38243044
-
An evolutionarily conserved strategy for ribosome binding and inhibition by β-coronavirus non-structural protein 1.bioRxiv [Preprint]. 2023 Jun 8:2023.06.07.544141. doi: 10.1101/2023.06.07.544141. bioRxiv. 2023. Update in: J Mol Biol. 2023 Oct 15;435(20):168259. doi: 10.1016/j.jmb.2023.168259 PMID: 37333070 Free PMC article. Updated. Preprint.
References
-
- Zaki AM, van Boheemen S, Bestebroer TM, et al. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367(19):1814–1820. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
