Synthesis and preliminary evaluation of octreotate conjugates of bioactive synthetic amatoxins for targeting somatostatin receptor (sstr2) expressing cells
- PMID: 35128410
- PMCID: PMC8729174
- DOI: 10.1039/d1cb00036e
Synthesis and preliminary evaluation of octreotate conjugates of bioactive synthetic amatoxins for targeting somatostatin receptor (sstr2) expressing cells
Abstract
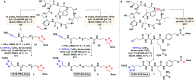
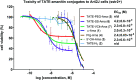
Targeted cancer therapy represents a paradigm-shifting approach that aims to deliver a toxic payload selectively to target-expressing cells thereby sparing normal tissues the off-target effects associated with traditional chemotherapeutics. Since most targeted constructs rely on standard microtubule inhibitors or DNA-reactive molecules as payloads, new toxins that inhibit other intracellular targets are needed to realize the full potential of targeted therapy. Among these new payloads, α-amanitin has gained attraction as a payload in targeted therapy. Here, we conjugate two synthetic amanitins at different sites to demonstrate their utility as payloads in peptide drug conjugates (PDCs). As an exemplary targeting agent, we chose octreotate, a well-studied somatostatin receptor (sstr2) peptide agonist for the conjugation to synthetic amatoxins via three tailor-built linkers. The linker chemistry permitted the evaluation of one non-cleavable and two cleavable self-immolative conjugates. The immolating linkers were chosen to take advantage of either the reducing potential of the intracellular environment or the high levels of lysosomal proteases in tumor cells to trigger toxin release. Cell-based assays on target-positive Ar42J cells revealed target-specific reduction in viability with up to 1000-fold enhancement in bioactivity compared to the untargeted amatoxins. Altogether, this preliminary study enabled the development of a highly modular synthetic platform for the construction of amanitin-based conjugates that can be readily extended to various targeting moieties.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

Similar articles
-
Discovery of an SSTR2-Targeting Maytansinoid Conjugate (PEN-221) with Potent Activity in Vitro and in Vivo.J Med Chem. 2019 Mar 14;62(5):2708-2719. doi: 10.1021/acs.jmedchem.8b02036. Epub 2019 Feb 28. J Med Chem. 2019. PMID: 30735385
-
Synthesis, drug release, and biological evaluation of new anticancer drug-bioconjugates containing somatostatin backbone cyclic analog as a targeting moiety.Biopolymers. 2015 Nov;104(6):743-52. doi: 10.1002/bip.22694. Biopolymers. 2015. PMID: 26058565
-
Octreotide Conjugates for Tumor Targeting and Imaging.Pharmaceutics. 2019 May 7;11(5):220. doi: 10.3390/pharmaceutics11050220. Pharmaceutics. 2019. PMID: 31067748 Free PMC article.
-
Somatostatin receptor-targeted anti-cancer therapy.Curr Drug Deliv. 2011 Jan;8(1):2-10. doi: 10.2174/156720111793663633. Curr Drug Deliv. 2011. PMID: 21034425 Review.
-
The Chemical Design and Synthesis of Linkers Used in Antibody Drug Conjugates.Curr Top Med Chem. 2017;17(32):3393-3424. doi: 10.2174/1568026618666180118155847. Curr Top Med Chem. 2017. PMID: 29357801 Review.
Cited by
-
Peptide-Drug Conjugates: Design, Chemistry, and Drug Delivery System as a Novel Cancer Theranostic.ACS Pharmacol Transl Sci. 2024 Jan 24;7(2):309-334. doi: 10.1021/acsptsci.3c00269. eCollection 2024 Feb 9. ACS Pharmacol Transl Sci. 2024. PMID: 38357281 Free PMC article. Review.
-
Oxime-Linked Peptide-Daunomycin Conjugates as Good Tools for Selection of Suitable Homing Devices in Targeted Tumor Therapy: An Overview.Int J Mol Sci. 2024 Feb 3;25(3):1864. doi: 10.3390/ijms25031864. Int J Mol Sci. 2024. PMID: 38339141 Free PMC article. Review.
-
Research advances in peptide‒drug conjugates.Acta Pharm Sin B. 2023 Sep;13(9):3659-3677. doi: 10.1016/j.apsb.2023.02.013. Epub 2023 Feb 28. Acta Pharm Sin B. 2023. PMID: 37719380 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

