Cardiac Gene Therapy With Relaxin Receptor 1 Overexpression Protects Against Acute Myocardial Infarction
- PMID: 35128209
- PMCID: PMC8807852
- DOI: 10.1016/j.jacbts.2021.10.012
Cardiac Gene Therapy With Relaxin Receptor 1 Overexpression Protects Against Acute Myocardial Infarction
Abstract
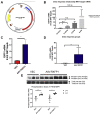
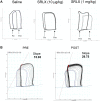
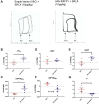
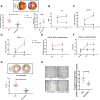
Relaxin is a pleiotropic hormone shown to confer cardioprotection in several preclinical models of cardiac ischemia-reperfusion injury. In the present study, the effects of up-regulating relaxin family peptide receptor 1 (RXFP1) via adeno-associated virus serotype 9 (AAV9) vectors were investigated in a mouse model of myocardial infarction. AAV9-RXFP1 vectors were generated and injected in adult male CD1 mice. Up-regulation of Rxfp1 was confirmed via quantitative polymerase chain reaction, and overexpressing animals showed increased sensitivity to relaxin-induced ventricular inotropic response. Overexpressing animals also demonstrated reduced infarct size and preserved cardiac function 24 hours after ischemia-reperfusion. Up-regulation of RXFP1 via AAV9 vectors has potential therapeutic utility in preventing adverse remodeling after myocardial infarction.
Keywords: AAV, adeno-associated virus; CMV, cytomegalovirus; GLS, global longitudinal strain; IR, ischemia-reperfusion; LV function; LV, left ventricular; MAPK, mitogen-activated protein kinase; MI, myocardial infarction; PV, pressure-volume; RXFP1; RXFP1, relaxin family peptide receptor 1; SIRO, simulated ischemia and reoxygenation; VEC, empty vector; eNOS, endothelial nitric oxide synthase; gene therapy; ischemia-reperfusion injury; mRNA, messenger ribonucleic acid; relaxin.
© 2022 The Authors.
Conflict of interest statement
This study was supported by the American Heart Association (grant 18PRE33990001 to Mr Devarakonda), the Wright Center for Clinical and Translational Research Center (Wright Scholarship to Mr Devarakonda), and the National Institutes of Health (grants R01HL142281, R21AG053654, R01HL133167, and R35HL155651 to Dr Salloum). The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures

Similar articles
-
B7-33, a Functionally Selective Relaxin Receptor 1 Agonist, Attenuates Myocardial Infarction-Related Adverse Cardiac Remodeling in Mice.J Am Heart Assoc. 2020 Apr 21;9(8):e015748. doi: 10.1161/JAHA.119.015748. Epub 2020 Apr 16. J Am Heart Assoc. 2020. PMID: 32295457 Free PMC article.
-
Reperfusion therapy with recombinant human relaxin-2 (Serelaxin) attenuates myocardial infarct size and NLRP3 inflammasome following ischemia/reperfusion injury via eNOS-dependent mechanism.Cardiovasc Res. 2017 May 1;113(6):609-619. doi: 10.1093/cvr/cvw246. Cardiovasc Res. 2017. PMID: 28073832
-
Ligand-activated RXFP1 gene therapy ameliorates pressure overload-induced cardiac dysfunction.Mol Ther. 2021 Aug 4;29(8):2499-2513. doi: 10.1016/j.ymthe.2021.04.010. Epub 2021 Apr 9. Mol Ther. 2021. PMID: 33839322 Free PMC article.
-
The relaxin family peptide receptor 1 (RXFP1): An emerging player in human health and disease.Mol Genet Genomic Med. 2020 Apr;8(4):e1194. doi: 10.1002/mgg3.1194. Epub 2020 Feb 26. Mol Genet Genomic Med. 2020. PMID: 32100955 Free PMC article. Review.
-
G-Protein-coupled receptors as potential drug candidates in preeclampsia: targeting the relaxin/insulin-like family peptide receptor 1 for treatment and prevention.Hum Reprod Update. 2016 Sep;22(5):647-64. doi: 10.1093/humupd/dmw021. Epub 2016 Jul 6. Hum Reprod Update. 2016. PMID: 27385360 Free PMC article. Review.
Cited by
-
Performance of Cardiotropic rAAV Vectors Is Dependent on Production Method.Viruses. 2022 Jul 26;14(8):1623. doi: 10.3390/v14081623. Viruses. 2022. PMID: 35893689 Free PMC article.
-
Appropriate Dose of Dapagliflozin Improves Cardiac Outcomes by Normalizing Mitochondrial Fission and Reducing Cardiomyocyte Apoptosis After Acute Myocardial Infarction.Drug Des Devel Ther. 2022 Jun 28;16:2017-2030. doi: 10.2147/DDDT.S371506. eCollection 2022. Drug Des Devel Ther. 2022. PMID: 35789742 Free PMC article.
-
Cardioprotection Achieved Through Overexpression of Relaxin Receptors.JACC Basic Transl Sci. 2022 Jan 24;7(1):64-66. doi: 10.1016/j.jacbts.2021.11.008. eCollection 2022 Jan. JACC Basic Transl Sci. 2022. PMID: 35128210 Free PMC article.
-
Expression characteristics of lipid metabolism-related genes and correlative immune infiltration landscape in acute myocardial infarction.Sci Rep. 2024 Jun 18;14(1):14095. doi: 10.1038/s41598-024-65022-3. Sci Rep. 2024. PMID: 38890389 Free PMC article.
References
-
- Bathgate R.A.D., Halls M.L., van der Westhuizen E.T., Callander G.E., Kocan M., Summers R.J. Relaxin family peptides and their receptors. Physiol Rev. 2013;93(1):405–480. - PubMed
-
- Halls M.L., Bathgate R.A.D., Sutton S.W., Dschietzig T.B., Summers R.J. International Union of Basic and Clinical Pharmacology. XCV. Recent advances in the understanding of the pharmacology and biological roles of relaxin family peptide receptors 1-4, the receptors for relaxin family peptides. Pharmacol Rev. 2015;67(2):389–440. - PMC - PubMed
-
- Masini E., Bani D., Bello M.G., Bigazzi M., Mannaioni P.F., Sacchi T.B. Relaxin counteracts myocardial damage induced by ischemia-reperfusion in isolated guinea pig hearts: evidence for an involvement of nitric oxide. Endocrinology. 1997;138(11):4713–4720. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

