Hepatitis C: Problems to extinction and residual hepatic and extrahepatic lesions after sustained virological response
- PMID: 35126840
- PMCID: PMC8790402
- DOI: 10.4254/wjh.v14.i1.62
Hepatitis C: Problems to extinction and residual hepatic and extrahepatic lesions after sustained virological response
Abstract
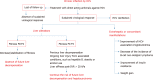
Loss of follow-up or reinfections hinder the expectations of hepatitis C eradication despite the existence of highly effective treatments. Moreover, the elimination of the infection does not imply the reversion of those chronic alterations derived from the previous infection by hepatitis C virus (HCV). This review analyzes the risk factors associated with loss to follow-up in diagnosis or treatment, and the possibility of reinfection. Likewise, it assesses the residual alterations induced by chronic HCV infection considering the liver alterations (inflammation, fibrosis, risk of decompensation, hepatocellular carcinoma, liver transplantation) and, on the other hand, the comorbidities and extrahepatic manifestations (cryoglobulinemia, non-Hodgkin lymphoma, peripheral insulin resistance, and lipid, bone and cognitive alterations). Peculiarities present in subjects coinfected with human immunodeficiency virus are analyzed in each section.
Keywords: Cirrhosis decompensation; Direct antiviral agents; Extrahepatic complications; Hepatitis C virus; Hepatocarcinoma; Human immunodeficiency virus; Sustained virological response.
©The Author(s) 2022. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: All authors reported no conflicts.
Figures
Similar articles
-
Burden, Outcome, and Comorbidities of Extrahepatic Manifestations in Hepatitis C Virus Infection.Biology (Basel). 2022 Dec 22;12(1):23. doi: 10.3390/biology12010023. Biology (Basel). 2022. PMID: 36671716 Free PMC article. Review.
-
Human Immunodeficiency Virus (HIV) Infection Is Associated With Lower Risk of Hepatocellular Carcinoma After Sustained Virological Response to Direct-acting Antivirals in Hepatitis C Infected Patients With Advanced Fibrosis.Clin Infect Dis. 2021 Oct 5;73(7):e2109-e2116. doi: 10.1093/cid/ciaa1111. Clin Infect Dis. 2021. PMID: 32766891
-
Reversion of disease manifestations after HCV eradication.J Hepatol. 2016 Oct;65(1 Suppl):S95-S108. doi: 10.1016/j.jhep.2016.07.039. J Hepatol. 2016. PMID: 27641991 Review.
-
Advanced liver disease outcomes after hepatitis C eradication by human immunodeficiency virus infection in PITER cohort.Hepatol Int. 2020 May;14(3):362-372. doi: 10.1007/s12072-020-10034-0. Epub 2020 Apr 11. Hepatol Int. 2020. PMID: 32279177 Free PMC article.
-
Extrahepatic morbidity and mortality of chronic hepatitis C.Gastroenterology. 2015 Nov;149(6):1345-60. doi: 10.1053/j.gastro.2015.08.035. Epub 2015 Aug 28. Gastroenterology. 2015. PMID: 26319013 Review.
Cited by
-
Prevention in Hepatology.J Pers Med. 2024 Jan 23;14(2):132. doi: 10.3390/jpm14020132. J Pers Med. 2024. PMID: 38392566 Free PMC article. Review.
-
Role of hepatitis c virus in hepatocellular carcinoma and neurological disorders: an overview.Front Oncol. 2022 Jul 29;12:913231. doi: 10.3389/fonc.2022.913231. eCollection 2022. Front Oncol. 2022. PMID: 35965577 Free PMC article. Review.
-
Hepatitis C and Thalassemia: A Story with (Almost) a Happy Ending.Pathogens. 2023 May 5;12(5):683. doi: 10.3390/pathogens12050683. Pathogens. 2023. PMID: 37242353 Free PMC article. Review.
References
-
- Polaris Observatory HCV Collaborators. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: A modelling study. Lancet Gastroenterol Hepatol. 2017;2:161–176. - PubMed
-
- Gottwein JM, Pham LV, Mikkelsen LS, Ghanem L, Ramirez S, Scheel TKH, Carlsen THR, Bukh J. Efficacy of NS5A inhibitors against hepatitis c virus genotypes 1-7 and escape variants. Gastroenterology. 2018;154:1435–1448. - PubMed
-
- World Health Organization. Guidelines for the care and treatment of persons diagnosed with chronic hepatitis C virus infection 2018. [cited 30 December 2020]. Available from: https://www.who.int/hepatitis/publications/hepatitis-c-guidelines-2018/en/ - PubMed
-
- AASLD/IDSA HCV guidance: Recommendations for testing, managing, and treating hepatitis C. Clin Liver Dis . 2018;12:117–117.
-
- European Association for the Study of the Liver. EASL Recommendations on Treatment of Hepatitis C 2018. J Hepatol. 2018;69:461–511. - PubMed

