Specific Interleukin-1 Inhibitors, Specific Interleukin-6 Inhibitors, and GM-CSF Blockades for COVID-19 (at the Edge of Sepsis): A Systematic Review
- PMID: 35126138
- PMCID: PMC8815770
- DOI: 10.3389/fphar.2021.804250
Specific Interleukin-1 Inhibitors, Specific Interleukin-6 Inhibitors, and GM-CSF Blockades for COVID-19 (at the Edge of Sepsis): A Systematic Review
Abstract
Sepsis is a syndrome with high mortality, which seriously threatens human health. During the pandemic of coronavirus disease 2019 (COVID-19), some severe and critically ill COVID-19 patients with multiple organ dysfunction developed characteristics typical of sepsis and met the diagnostic criteria for sepsis. Timely detection of cytokine storm and appropriate regulation of inflammatory response may be significant in the prevention and treatment of sepsis. This study evaluated the efficacy and safety of specific interleukin (IL)-1 inhibitors, specific IL-6 inhibitors, and GM-CSF blockades in the treatment of COVID-19 (at the edge of sepsis) patients through systematic review and meta-analysis.
Methodology: A literature search was conducted on PubMed, EMBASE, Clinical Key, Cochrane Library, CNKI, and Wanfang Database using proper keywords such as "SARS-CoV-2," "Corona Virus Disease 2019," "COVID-19," "anakinra," "tocilizumab," "siltuximab," "sarilumab," "mavrilimumab," "lenzilumab," and related words for publications released until August 22, 2021. Other available resources were also used to identify relevant articles. The present systematic review was performed based on PRISMA protocol.
Results: Based on the inclusion and exclusion criteria, 43 articles were included in the final review. The meta-analysis results showed that tocilizumab could reduce the mortality of patients with COVID-19 (at the edge of sepsis) [randomized controlled trials, RCTs: odds ratio (OR) 0.71, 95%CI: 0.52-0.97, low-certainty evidence; non-RCTs: risk ratio (RR) 0.68, 95%CI: 0.55-0.84, very low-certainty evidence) as was anakinra (non-RCTs: RR 0.47, 95%CI: 0.34-0.66, very low-certainty evidence). Sarilumab might reduce the mortality of patients with COVID-19 (at the edge of sepsis), but there was no statistical significance (OR 0.65, 95%CI: 0.36-1.2, low-certainty evidence). For safety outcomes, whether tocilizumab had an impact on serious adverse events (SAEs) was very uncertain (RCTs: OR 0.87, 95%CI: 0.38-2.0, low-certainty evidence; non-RCTs 1.18, 95%CI: 0.83-1.68, very low-certainty evidence) as was on secondary infections (RCTs: OR 0.71, 95%CI: 0.06-8.75, low-certainty evidence; non-RCTs: RR 1.15, 95%CI: 0.89-1.49, very low-certainty evidence).
Conclusions: This systematic review showed that tocilizumab, sarilumab, and anakinra could reduce the mortality of people with COVID-19 (at the edge of sepsis), and tocilizumab did not significantly affect SAEs and secondary infections. The current evidence of the studies on patients treated with siltuximab, mavrilimumab, and lenzilumab is insufficient. In order to establish evidence with stronger quality, high-quality studies are needed. Systematic Review Registration: PROSPERO (https://www.crd.york.ac.uk/prospero/), identifier CRD42020226545.
Keywords: GM-CSF blockades; SARS-CoV-2; coronavirus disease 2019 (COVID-19); sepsis; specific interleukin-1 inhibitors; specific interleukin-6 inhibitors.
Copyright © 2022 Wang, Zhu, Dai, Li, Li, Lv and Yu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


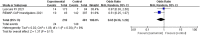
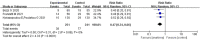


Similar articles
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a living systematic review.Cochrane Database Syst Rev. 2020 Oct 12;10:CD013600. doi: 10.1002/14651858.CD013600.pub3. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 May 20;5:CD013600. doi: 10.1002/14651858.CD013600.pub4. PMID: 33044747 Updated.
-
Unraveling the impact of nitric oxide, almitrine, and their combination in COVID-19 (at the edge of sepsis) patients: a systematic review.Front Pharmacol. 2024 Jan 22;14:1172447. doi: 10.3389/fphar.2023.1172447. eCollection 2023. Front Pharmacol. 2024. PMID: 38318311 Free PMC article.
-
Interleukin-1 blocking agents for treating COVID-19.Cochrane Database Syst Rev. 2022 Jan 26;1(1):CD015308. doi: 10.1002/14651858.CD015308. Cochrane Database Syst Rev. 2022. PMID: 35080773 Free PMC article. Review.
-
SARS-CoV-2-neutralising monoclonal antibodies to prevent COVID-19.Cochrane Database Syst Rev. 2022 Jun 17;6(6):CD014945. doi: 10.1002/14651858.CD014945.pub2. Cochrane Database Syst Rev. 2022. PMID: 35713300 Free PMC article. Review.
Cited by
-
Neutrophils and the Systemic Inflammatory Response Syndrome (SIRS).Int J Mol Sci. 2023 Aug 30;24(17):13469. doi: 10.3390/ijms241713469. Int J Mol Sci. 2023. PMID: 37686271 Free PMC article. Review.
-
Protective effects of Jing-Si-herbal-tea in inflammatory cytokines-induced cell injury on normal human lung fibroblast via multiomic platform analysis.Tzu Chi Med J. 2024 Mar 26;36(2):152-165. doi: 10.4103/tcmj.tcmj_267_23. eCollection 2024 Apr-Jun. Tzu Chi Med J. 2024. PMID: 38645788 Free PMC article.
-
Ameliorating effects of berberine on sepsis-associated lung inflammation induced by lipopolysaccharide: molecular mechanisms and preclinical evidence.Pharmacol Rep. 2023 Aug;75(4):805-816. doi: 10.1007/s43440-023-00492-2. Epub 2023 May 15. Pharmacol Rep. 2023. PMID: 37184743 Review.
-
The Impact of Cytokines on Neutrophils' Phagocytosis and NET Formation during Sepsis-A Review.Int J Mol Sci. 2022 May 3;23(9):5076. doi: 10.3390/ijms23095076. Int J Mol Sci. 2022. PMID: 35563475 Free PMC article. Review.
-
Coronavirus Infection 2019 (COVID-19) and Autoimmunity.Her Russ Acad Sci. 2022;92(4):398-403. doi: 10.1134/S1019331622040062. Epub 2022 Sep 6. Her Russ Acad Sci. 2022. PMID: 36091857 Free PMC article.
References
-
- Bernardo L., Del Sesto S., Giordano L., Benincaso A. R., Biondi P., Goj V., et al. (2020). Severe Prolonged Neutropenia Following Administration of Tocilizumab in a Patient Affected by COVID-19: a Case Report and Brief Review of the Literature. Drugs Ther. Perspect. 14, 1–5. 10.1007/s40267-020-00777-z - DOI - PMC - PubMed
-
- Bozzi G., Mangioni D., Minoia F., Aliberti S., Grasselli G., Barbetta L., et al. (2021). Anakinra Combined With Methylprednisolone in Patients With Severe COVID-19 Pneumonia and Hyperinflammation: An Observational Cohort Study. J. Allergy Clin. Immunol. 147 (2), 561–e4. e4. 10.1016/j.jaci.2020.11.006 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

