Membrane progesterone receptor α (mPRα) enhances hypoxia-induced vascular endothelial growth factor secretion and angiogenesis in lung adenocarcinoma through STAT3 signaling
- PMID: 35123491
- PMCID: PMC8817580
- DOI: 10.1186/s12967-022-03270-5
Membrane progesterone receptor α (mPRα) enhances hypoxia-induced vascular endothelial growth factor secretion and angiogenesis in lung adenocarcinoma through STAT3 signaling
Abstract
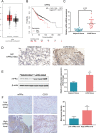
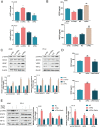
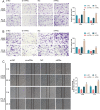
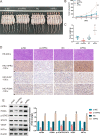
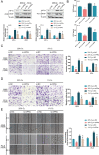
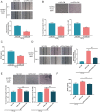
Lung cancer remains a huge challenge to public health because of its high incidence and mortality, and lung adenocarcinoma (LUAD) is the main subtype of lung cancer. Hypoxia-induced vascular endothelial growth factor (VEGF) release and angiogenesis have been regarded as critical events in LUAD carcinogenesis. In the present study, membrane progesterone receptor α (mPRα) is deregulated within LUAD tissue samples; increased mPRα contributes to a higher microvessel density (MVD) in LUAD tissues. mPRα knockdown in A549 and PC-9 cells significantly inhibited STAT3 phosphorylation, as well as HIF1α and VEGF protein levels, decreasing cancer cell migration and invasion. The in vivo xenograft model further confirmed that mPRα enhanced the aggressiveness of LUAD cells. Furthermore, mPRα knockdown significantly inhibited hypoxia-induced upregulation in HIF1α and VEGF levels, as well as LUAD cell migration and invasion. Under the hypoxic condition, conditioned medium (CM) derived from mPRα knockdown A549 cells, namely si-mPRα-CM, significantly inhibited HUVEC migration and tube formation and decreased VEGF level in the culture medium. In contrast, CM derived from mPRα-overexpressing A549 cells, namely mPRα-CM, further enhanced HUVEC migration and tube formation and increased VEGF level under hypoxia, which was partially reversed by STAT3 inhibitor Stattic. In conclusion, in LUAD cells, highly expressed mPRα enhances the activation of cAMP/JAK/STAT3 signaling and increases HIF1α-induced VEGF secretion into the tumor microenvironment, promoting HUVEC migration and tube formation under hypoxia.
Keywords: Angiogenesis; Hypoxia; Lung adenocarcinoma; Membrane progesterone receptor α (mPRα); STAT3 signaling; Vascular endothelial growth factor (VEGF).
© 2022. The Author(s).
Conflict of interest statement
None.
Figures
Similar articles
-
TMEM9 promotes lung adenocarcinoma progression via activating the MEK/ERK/STAT3 pathway to induce VEGF expression.Cell Death Dis. 2024 Apr 25;15(4):295. doi: 10.1038/s41419-024-06669-8. Cell Death Dis. 2024. PMID: 38664392 Free PMC article.
-
The mineral dust-induced gene, mdig, regulates angiogenesis and lymphangiogenesis in lung adenocarcinoma by modulating the expression of VEGF-A/C/D via EGFR and HIF-1α signaling.Oncol Rep. 2021 May;45(5):60. doi: 10.3892/or.2021.8011. Epub 2021 Mar 24. Oncol Rep. 2021. PMID: 33760153
-
MicroRNA-204 deregulation in lung adenocarcinoma controls the biological behaviors of endothelial cells potentially by modulating Janus kinase 2-signal transducer and activator of transcription 3 pathway.IUBMB Life. 2018 Jan;70(1):81-91. doi: 10.1002/iub.1706. IUBMB Life. 2018. PMID: 29281186
-
Chinese Herbal Compound Xiaoliu Pingyi Recipe Inhibits the Growth of Lung Adenocarcinoma by Regulating the Tumor Vascular Microenvironment.Integr Cancer Ther. 2024 Jan-Dec;23:15347354241273962. doi: 10.1177/15347354241273962. Integr Cancer Ther. 2024. PMID: 39223822 Free PMC article.
-
Cell Type-Specific Roles of STAT3 Signaling in the Pathogenesis and Progression of K-ras Mutant Lung Adenocarcinoma.Cancers (Basel). 2022 Mar 31;14(7):1785. doi: 10.3390/cancers14071785. Cancers (Basel). 2022. PMID: 35406557 Free PMC article. Review.
Cited by
-
Expression of lncRNA LINC00943 in lung squamous cell carcinoma and its relationship with tumor progression.J Cardiothorac Surg. 2024 Apr 16;19(1):222. doi: 10.1186/s13019-024-02771-2. J Cardiothorac Surg. 2024. PMID: 38627774 Free PMC article.
-
CmPn/CmP Signaling Networks in the Maintenance of the Blood Vessel Barrier.J Pers Med. 2023 Apr 28;13(5):751. doi: 10.3390/jpm13050751. J Pers Med. 2023. PMID: 37240921 Free PMC article. Review.
-
Complement factor H inhibits endothelial cell migration through suppression of STAT3 signaling.Exp Ther Med. 2023 Jul 10;26(2):408. doi: 10.3892/etm.2023.12107. eCollection 2023 Aug. Exp Ther Med. 2023. PMID: 37522066 Free PMC article.
-
New Perspectives on Sex Steroid Hormones Signaling in Cancer-Associated Fibroblasts of Non-Small Cell Lung Cancer.Cancers (Basel). 2023 Jul 14;15(14):3620. doi: 10.3390/cancers15143620. Cancers (Basel). 2023. PMID: 37509283 Free PMC article. Review.
-
Angiogenesis modulated by CD93 and its natural ligands IGFBP7 and MMRN2: a new target to facilitate solid tumor therapy by vasculature normalization.Cancer Cell Int. 2023 Sep 2;23(1):189. doi: 10.1186/s12935-023-03044-z. Cancer Cell Int. 2023. PMID: 37660019 Free PMC article. Review.
References
-
- Torre LA, Siegel RL, Jemal A. Lung cancer statistics. Adv Exp Med Biol. 2016;893:1–19. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

