Survival of an HLA-mismatched, bioengineered RPE implant in dry age-related macular degeneration
- PMID: 35120620
- PMCID: PMC9039755
- DOI: 10.1016/j.stemcr.2022.01.001
Survival of an HLA-mismatched, bioengineered RPE implant in dry age-related macular degeneration
Abstract
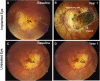
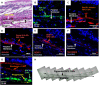
Cell-based therapies face challenges, including poor cell survival, immune rejection, and integration into pathologic tissue. We conducted an open-label phase 1/2a clinical trial to assess the safety and preliminary efficacy of a subretinal implant consisting of a polarized monolayer of allogeneic human embryonic stem cell-derived retinal pigmented epithelium (RPE) cells in subjects with geographic atrophy (GA) secondary to dry age-related macular degeneration. Postmortem histology from one subject with very advanced disease shows the presence of donor RPE cells 2 years after implantation by immunoreactivity for RPE65 and donor-specific human leukocyte antigen (HLA) class I molecules. Markers of RPE cell polarity and phagocytosis suggest donor RPE function. Further histologic examination demonstrated CD34+ structures beneath the implant and CD4+, CD68+, and FoxP3+ cells in the tissue. Despite significant donor-host HLA mismatch, no clinical signs of retinitis, vitreitis, vasculitis, choroiditis, or serologic immune response were detected in the deceased subject or any other subject in the study. Subretinally implanted, HLA-mismatched donor RPE cells survive, express functional markers, and do not elicit clinically detectable intraocular inflammation or serologic immune responses even without long-term immunosuppression.
Keywords: allogeneic; clinical trial; geographic atrophy; histology; implant; macular degeneration; parylene; polarized; retinal pigmented epithelium; stem cells.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Figures


Similar articles
-
A bioengineered retinal pigment epithelial monolayer for advanced, dry age-related macular degeneration.Sci Transl Med. 2018 Apr 4;10(435):eaao4097. doi: 10.1126/scitranslmed.aao4097. Sci Transl Med. 2018. PMID: 29618560 Clinical Trial.
-
Long-term Follow-up of a Phase 1/2a Clinical Trial of a Stem Cell-Derived Bioengineered Retinal Pigment Epithelium Implant for Geographic Atrophy.Ophthalmology. 2024 Jun;131(6):682-691. doi: 10.1016/j.ophtha.2023.12.028. Epub 2023 Dec 30. Ophthalmology. 2024. PMID: 38160882 Clinical Trial.
-
One-Year Follow-Up in a Phase 1/2a Clinical Trial of an Allogeneic RPE Cell Bioengineered Implant for Advanced Dry Age-Related Macular Degeneration.Transl Vis Sci Technol. 2021 Aug 12;10(10):13. doi: 10.1167/tvst.10.10.13. Transl Vis Sci Technol. 2021. PMID: 34613357 Free PMC article. Clinical Trial.
-
Induced pluripotent stem cell-based therapy for age-related macular degeneration.Expert Opin Biol Ther. 2017 Sep;17(9):1113-1126. doi: 10.1080/14712598.2017.1346079. Epub 2017 Jun 30. Expert Opin Biol Ther. 2017. PMID: 28664762 Review.
-
Stem Cell-Based Therapy for Diseases of the Retinal Pigment Epithelium: From Bench to Bedside.Semin Ophthalmol. 2016;31(1-2):25-9. doi: 10.3109/08820538.2015.1115253. Semin Ophthalmol. 2016. PMID: 26959126 Review.
Cited by
-
Polarized RPE Secretome Preserves Photoreceptors in Retinal Dystrophic RCS Rats.Cells. 2023 Jun 22;12(13):1689. doi: 10.3390/cells12131689. Cells. 2023. PMID: 37443724 Free PMC article.
-
Human pluripotent stem cells for the modelling of retinal pigment epithelium homeostasis and disease: A review.Clin Exp Ophthalmol. 2022 Aug;50(6):667-677. doi: 10.1111/ceo.14128. Epub 2022 Jul 11. Clin Exp Ophthalmol. 2022. PMID: 35739648 Free PMC article. Review.
-
Micromolded honeycomb scaffold design to support the generation of a bilayered RPE and photoreceptor cell construct.Bioact Mater. 2023 Jul 31;30:142-153. doi: 10.1016/j.bioactmat.2023.07.019. eCollection 2023 Dec. Bioact Mater. 2023. PMID: 37575875 Free PMC article.
-
Advances in the engineering of the outer blood-retina barrier: From in-vitro modelling to cellular therapy.Bioact Mater. 2023 Aug 12;31:151-177. doi: 10.1016/j.bioactmat.2023.08.003. eCollection 2024 Jan. Bioact Mater. 2023. PMID: 37637086 Free PMC article. Review.
-
Volumetric Reconstruction of a Human Retinal Pigment Epithelial Cell Reveals Specialized Membranes and Polarized Distribution of Organelles.Invest Ophthalmol Vis Sci. 2023 Dec 1;64(15):35. doi: 10.1167/iovs.64.15.35. Invest Ophthalmol Vis Sci. 2023. PMID: 38133501 Free PMC article.
References
-
- Algvere P.V., Berglin L., Gouras P., Sheng Y., Kopp E.D. Transplantation of RPE in age-related macular degeneration: observations in disciform lesions and dry RPE atrophy. Graefes Arch. Clin. Exp. Ophthalmol. 1997;235:149–158. - PubMed
-
- Benner J.D., Sunness J.S., Ziegler M.D., Soltanian J. Limited macular translocation for atrophic maculopathy. Arch. Ophthalmol. 2002;120:586–591. - PubMed
-
- Binder S., Krebs I., Hilgers R.D., Abri A., Stolba U., Assadoulina A., Kellner L., Stanzel B.V., Jahn C., Feichtinger H. Outcome of transplantation of autologous retinal pigment epithelium in age-related macular degeneration: a prospective trial. Invest. Ophthalmol. Vis. Sci. 2004;45:4151–4160. - PubMed
-
- Brant Fernandes R.A., Koss M.J., Falabella P., Stefanini F.R., Maia M., Diniz B., Ribeiro R., Hu Y., Hinton D., Clegg D.O., et al. An innovative surgical technique for subretinal transplantation of human embryonic stem cell-derived retinal pigmented epithelium in yucatan mini pigs: preliminary results. Ophthalmic Surg. Lasers Imaging Retina. 2016;47:342–351. - PubMed
-
- Cahill M.T., Mruthyunjaya P., Bowes Rickman C., Toth C.A. Recurrence of retinal pigment epithelial changes after macular translocation with 360 degrees peripheral retinectomy for geographic atrophy. Arch. Ophthalmol. 2005;123:935–938. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

