Fungal mycobiome drives IL-33 secretion and type 2 immunity in pancreatic cancer
- PMID: 35120601
- PMCID: PMC8847236
- DOI: 10.1016/j.ccell.2022.01.003
Fungal mycobiome drives IL-33 secretion and type 2 immunity in pancreatic cancer
Abstract
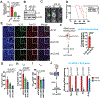
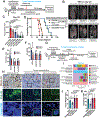
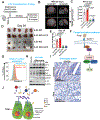
TH2 cells and innate lymphoid cells 2 (ILC2) can stimulate tumor growth by secreting pro-tumorigenic cytokines such as interleukin-4 (IL-4), IL-5, and IL-13. However, the mechanisms by which type 2 immune cells traffic to the tumor microenvironment are unknown. Here, we show that oncogenic KrasG12D increases IL-33 expression in pancreatic ductal adenocarcinoma (PDAC) cells, which recruits and activates TH2 and ILC2 cells. Correspondingly, cancer-cell-specific deletion of IL-33 reduces TH2 and ILC2 recruitment and promotes tumor regression. Unexpectedly, IL-33 secretion is dependent on the intratumoral fungal mycobiome. Genetic deletion of IL-33 or anti-fungal treatment decreases TH2 and ILC2 infiltration and increases survival. Consistently, high IL-33 expression is observed in approximately 20% of human PDAC, and expression is mainly restricted to cancer cells. These data expand our knowledge of the mechanisms driving PDAC tumor progression and identify therapeutically targetable pathways involving intratumoral mycobiome-driven secretion of IL-33.
Keywords: IL-33; ILC2; Kras; PDAC; TH2; anti-fungal therapy; fungal mycobiome; innate lymphoid cells; intratumor-microbiome; type 2 immune response.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests P. Dey and A.A. have a patent pending on targeting IL-33 and mycobiome in cancer: US Provisional Patent Application Serial No. 63/238,531. R.A.D. is a co-founder, adviser, and/or director of Tvardi Therapeutics, Asylia Therapeutics, Stellanova Therapeutics, Nirogy Therapeutics, and Sporos Bioventures. Tvardi and Nirogy are developing STAT3 and MCT inhibitors, respectively. A.M. receives royalties from Cosmos Wisdom Biotechnology and Thrive Earlier Detection, an Exact Sciences Company. A.M. is also a consultant for Freenome and Tezcat Biotechnology. The other authors declare no competing interests.
Figures



Comment in
-
The mycobiome-immune axis: The next frontier in pancreatic cancer.Cancer Cell. 2022 Feb 14;40(2):120-122. doi: 10.1016/j.ccell.2022.01.009. Cancer Cell. 2022. PMID: 35167821 Free PMC article.
Similar articles
-
T-cell programming in pancreatic adenocarcinoma: a review.Cancer Gene Ther. 2017 Mar;24(3):106-113. doi: 10.1038/cgt.2016.66. Epub 2016 Dec 2. Cancer Gene Ther. 2017. PMID: 27910859 Review.
-
T cells are necessary for ILC2 activation in house dust mite-induced allergic airway inflammation in mice.Eur J Immunol. 2016 Jun;46(6):1392-403. doi: 10.1002/eji.201546119. Epub 2016 May 12. Eur J Immunol. 2016. PMID: 27062360
-
Patched 1-interacting Peptide Represses Fibrosis in Pancreatic Cancer to Augment the Effectiveness of Immunotherapy.J Immunother. 2020 May;43(4):121-133. doi: 10.1097/CJI.0000000000000305. J Immunother. 2020. PMID: 31834207
-
Single-cell RNA sequencing reveals compartmental remodeling of tumor-infiltrating immune cells induced by anti-CD47 targeting in pancreatic cancer.J Hematol Oncol. 2019 Nov 27;12(1):124. doi: 10.1186/s13045-019-0822-6. J Hematol Oncol. 2019. PMID: 31771616 Free PMC article.
-
The reciprocal regulation between host tissue and immune cells in pancreatic ductal adenocarcinoma: new insights and therapeutic implications.Mol Cancer. 2019 Dec 13;18(1):184. doi: 10.1186/s12943-019-1117-9. Mol Cancer. 2019. PMID: 31831007 Free PMC article. Review.
Cited by
-
Inflammatory Processes: Key Mediators of Oncogenesis and Progression in Pancreatic Ductal Adenocarcinoma (PDAC).Int J Mol Sci. 2024 Oct 12;25(20):10991. doi: 10.3390/ijms252010991. Int J Mol Sci. 2024. PMID: 39456771 Free PMC article. Review.
-
Tertiary Lymphoid Structures in Microorganism-Related Cancer.Cancers (Basel). 2024 Oct 12;16(20):3464. doi: 10.3390/cancers16203464. Cancers (Basel). 2024. PMID: 39456558 Free PMC article. Review.
-
Effects of intratumoral microbiota on tumorigenesis, anti-tumor immunity, and microbe-based cancer therapy.Front Oncol. 2024 Sep 26;14:1429722. doi: 10.3389/fonc.2024.1429722. eCollection 2024. Front Oncol. 2024. PMID: 39391251 Free PMC article. Review.
-
Tumor microenvironment as a complex milieu driving cancer progression: a mini review.Clin Transl Oncol. 2024 Sep 28. doi: 10.1007/s12094-024-03697-w. Online ahead of print. Clin Transl Oncol. 2024. PMID: 39342061 Review.
-
The type 2 cytokine Fc-IL-4 revitalizes exhausted CD8+ T cells against cancer.Nature. 2024 Oct;634(8034):712-720. doi: 10.1038/s41586-024-07962-4. Epub 2024 Sep 25. Nature. 2024. PMID: 39322665 Free PMC article.
References
-
- ABARENKOV K, HENRIK NILSSON R, LARSSON KH, ALEXANDER IJ, EBERHARDT U, ERLAND S, HOILAND K, KJOLLER R, LARSSON E, PENNANEN T, SEN R, TAYLOR AF, TEDERSOO L, URSING BM, VRALSTAD T, LIIMATAINEN K, PEINTNER U & KOLJALG U 2010. The UNITE database for molecular identification of fungi--recent updates and future perspectives. New Phytol, 186, 281–5. - PubMed
-
- ALONSO-CURBELO D, HO YJ, BURDZIAK C, MAAG JLV, MORRIS JPT, CHANDWANI R, CHEN HA, TSANOV KM, BARRIGA FM, LUAN W, TASDEMIR N, LIVSHITS G, AZIZI E, CHUN J, WILKINSON JE, MAZUTIS L, LEACH SD, KOCHE R, PE’ER D & LOWE SW 2021. A gene-environment-induced epigenetic program initiates tumorigenesis. Nature, 590, 642–648. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

