Cellular ESCRT components are recruited to regulate the endocytic trafficking and RNA replication compartment assembly during classical swine fever virus infection
- PMID: 35120190
- PMCID: PMC8849529
- DOI: 10.1371/journal.ppat.1010294
Cellular ESCRT components are recruited to regulate the endocytic trafficking and RNA replication compartment assembly during classical swine fever virus infection
Abstract
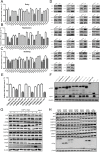
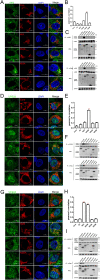
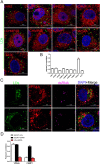
As the important molecular machinery for membrane protein sorting in eukaryotic cells, the endosomal sorting and transport complexes (ESCRT-0/I/II/III and VPS4) usually participate in various replication stages of enveloped viruses, such as endocytosis and budding. The main subunit of ESCRT-I, Tsg101, has been previously revealed to play a role in the entry and replication of classical swine fever virus (CSFV). However, the effect of the whole ESCRT machinery during CSFV infection has not yet been well defined. Here, we systematically determine the effects of subunits of ESCRT on entry, replication, and budding of CSFV by genetic analysis. We show that EAP20 (VPS25) (ESCRT-II), CHMP4B and CHMP7 (ESCRT-III) regulate CSFV entry and assist vesicles in transporting CSFV from Clathrin, early endosomes, late endosomes to lysosomes. Importantly, we first demonstrate that HRS (ESCRT-0), VPS28 (ESCRT-I), VPS25 (ESCRT-II) and adaptor protein ALIX play important roles in the formation of virus replication complexes (VRC) together with CHMP2B/4B/7 (ESCRT-III), and VPS4A. Further analyses reveal these subunits interact with CSFV nonstructural proteins (NS) and locate in the endoplasmic reticulum, but not Golgi, suggesting the role of ESCRT in regulating VRC assembly. In addition, we demonstrate that VPS4A is close to lipid droplets (LDs), indicating the importance of lipid metabolism in the formation of VRC and nucleic acid production. Altogether, we draw a new picture of cellular ESCRT machinery in CSFV entry and VRC formation, which could provide alternative strategies for preventing and controlling the diseases caused by CSFV or other Pestivirus.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Attachment, Entry, and Intracellular Trafficking of Classical Swine Fever Virus.Viruses. 2023 Sep 3;15(9):1870. doi: 10.3390/v15091870. Viruses. 2023. PMID: 37766277 Free PMC article. Review.
-
The Small GTPase Rab14 Regulates the Trafficking of Ceramide from Endoplasmic Reticulum to Golgi Apparatus and Facilitates Classical Swine Fever Virus Assembly.J Virol. 2023 May 31;97(5):e0036423. doi: 10.1128/jvi.00364-23. Epub 2023 Apr 12. J Virol. 2023. PMID: 37255314 Free PMC article.
-
The ESCRT-I Subunit Tsg101 Plays Novel Dual Roles in Entry and Replication of Classical Swine Fever Virus.J Virol. 2021 Feb 24;95(6):e01928-20. doi: 10.1128/JVI.01928-20. Print 2021 Feb 24. J Virol. 2021. PMID: 33328308 Free PMC article.
-
Fatty Acid Synthase Is Involved in Classical Swine Fever Virus Replication by Interaction with NS4B.J Virol. 2021 Aug 10;95(17):e0078121. doi: 10.1128/JVI.00781-21. Epub 2021 Aug 10. J Virol. 2021. PMID: 34132567 Free PMC article.
-
[Research Progress in the Core Proteins of the Classical Swine Fever Virus].Bing Du Xue Bao. 2015 Sep;31(5):579-84. Bing Du Xue Bao. 2015. PMID: 26738299 Review. Chinese.
Cited by
-
Attachment, Entry, and Intracellular Trafficking of Classical Swine Fever Virus.Viruses. 2023 Sep 3;15(9):1870. doi: 10.3390/v15091870. Viruses. 2023. PMID: 37766277 Free PMC article. Review.
-
The Small GTPase Rab14 Regulates the Trafficking of Ceramide from Endoplasmic Reticulum to Golgi Apparatus and Facilitates Classical Swine Fever Virus Assembly.J Virol. 2023 May 31;97(5):e0036423. doi: 10.1128/jvi.00364-23. Epub 2023 Apr 12. J Virol. 2023. PMID: 37255314 Free PMC article.
-
Mutation of Phenylalanine 23 of Newcastle Disease Virus Matrix Protein Inhibits Virus Release by Disrupting the Interaction between the FPIV L-Domain and Charged Multivesicular Body Protein 4B.Microbiol Spectr. 2023 Feb 14;11(1):e0411622. doi: 10.1128/spectrum.04116-22. Epub 2023 Jan 25. Microbiol Spectr. 2023. PMID: 36695580 Free PMC article.
-
Classical swine fever virus non-structural protein 4A recruits dihydroorotate dehydrogenase to facilitate viral replication.J Virol. 2024 Jun 13;98(6):e0049424. doi: 10.1128/jvi.00494-24. Epub 2024 May 17. J Virol. 2024. PMID: 38757985 Free PMC article.
-
Insights into the function of ESCRT and its role in enveloped virus infection.Front Microbiol. 2023 Oct 6;14:1261651. doi: 10.3389/fmicb.2023.1261651. eCollection 2023. Front Microbiol. 2023. PMID: 37869652 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

