Atezolizumab-induced psoriasis in a patient with metastatic lung cancer-a case report
- PMID: 35117743
- PMCID: PMC8798521
- DOI: 10.21037/tcr.2020.03.57
Atezolizumab-induced psoriasis in a patient with metastatic lung cancer-a case report
Abstract
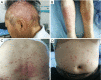
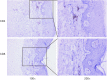
Immune checkpoint inhibitors, now FDA-approved, are being increasingly used for diverse cancer types. Dermatological complications are most frequent immune-related adverse events during immune checkpoints inhibitors therapies. There is no case reporting psoriasis exacerbation from Asia until now. We present a case of a 53-year-old Chinese man with non-small cell lung cancer (NSCLC) who presented with severe psoriasis at about two weeks after atezolizumab initiation. A skin punch biopsy was performed which revealed these were hyperkeratosis with IL-17A expression positive and confirmed the diagnosis of psoriasis. Atezolizumab was discontinued. Psoriasis was treated with flumethasone ointment every 12 hours and desloratadine 5 mg once daily for 2 weeks instead of phototherapies and improved completely over the next 2 months. He received chemotherapy in 4 cycles and radiotherapy and remained stable disease until December, 2019. Oncologists should pay attention to potential psoriasis exacerbation when patients use anti-programmed death ligand 1 (anti-PD-L1), especially who had a personal psoriasis-related history.
Keywords: Atezolizumab; anti-programmed death ligand 1 (anti-PD-L1); case report; non-small cell lung cancer (NSCLC); psoriasis.
2020 Translational Cancer Research. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.03.57). The authors have no conflicts of interest to declare.
Figures



Similar articles
-
Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial.Lancet. 2016 Apr 30;387(10030):1837-46. doi: 10.1016/S0140-6736(16)00587-0. Epub 2016 Mar 10. Lancet. 2016. PMID: 26970723 Clinical Trial.
-
New PD-L1 inhibitors in non-small cell lung cancer - impact of atezolizumab.Lung Cancer (Auckl). 2017 Jul 13;8:67-78. doi: 10.2147/LCTT.S113177. eCollection 2017. Lung Cancer (Auckl). 2017. PMID: 28761384 Free PMC article. Review.
-
Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial.Lancet. 2017 Jan 21;389(10066):255-265. doi: 10.1016/S0140-6736(16)32517-X. Epub 2016 Dec 13. Lancet. 2017. PMID: 27979383 Free PMC article. Clinical Trial.
-
Biological Agents for Treating Atezolizumab-Induced Psoriasis in Small-Cell Lung Cancer: A Case Report.Cureus. 2024 May 30;16(5):e61395. doi: 10.7759/cureus.61395. eCollection 2024 May. Cureus. 2024. PMID: 38947665 Free PMC article.
-
Immune-related organizing pneumonitis in non-small cell lung cancer receiving PD-1 inhibitor treatment: A case report and literature review.J Cancer Res Ther. 2020;16(7):1555-1559. doi: 10.4103/jcrt.JCRT_971_20. J Cancer Res Ther. 2020. PMID: 33565499 Review.
Cited by
-
Case report: Mycobacterium neoaurum infection during ICI therapy in a hepatocellular carcinoma patient with psoriasis.Front Immunol. 2022 Aug 22;13:972302. doi: 10.3389/fimmu.2022.972302. eCollection 2022. Front Immunol. 2022. PMID: 36072586 Free PMC article.
-
Perspectives on Psoriasiform Adverse Events from Immune Checkpoint Inhibitors: Lessons Learned from Our Practice.Medicina (Kaunas). 2024 Feb 22;60(3):373. doi: 10.3390/medicina60030373. Medicina (Kaunas). 2024. PMID: 38541099 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
