Paris polyphylla ethanol extract induces G2/M arrest and suppresses migration and invasion in bladder cancer
- PMID: 35117211
- PMCID: PMC8797771
- DOI: 10.21037/tcr-20-1512
Paris polyphylla ethanol extract induces G2/M arrest and suppresses migration and invasion in bladder cancer
Abstract
Background: Paris polyphylla is a traditional Chinese medicinal herb with multiple antitumor activities, but the role of P. polyphylla in bladder cancer (BC) is under investigation. This study aims to examine the antitumor activities of P. polyphylla ethanol extract (PPE) on BC cells and elucidate the underlying mechanisms.
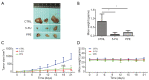
Methods: Viable cells were counted using the trypan blue exclusion assay. The cell cycle was analyzed using flow cytometry, and scratch wound-healing and transwell assays were used to evaluate cell migration and invasion abilities, respectively. The protein expression levels were determined by western blotting. A xenograft model was used to assess the in vivo inhibitory effect of PPE on BC tumor growth.
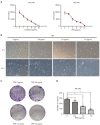
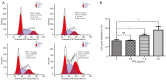
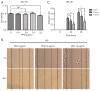
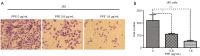
Results: Our results showed that PPE inhibited the growth of BC cells in vivo and in vitro. Mechanistically, PPE regulated the levels of cell cycle-associated proteins, with PPE-induced G2/M phase arrest occurring through cyclin-dependent kinase inhibitor 1 (CDKN1A) accumulation and cyclin B1 (CCNB1)/cyclin-dependent kinase 1 (CDK1) inhibition. BC tumor growth was also inhibited by PPE treatment. Moreover, the migration and invasion abilities of J82 cells were suppressed through modulating epithelial-mesenchymal transition (EMT) regulatory factors with upregulation of cadherin-1 (CDH1) and downregulation of cadherin-2 (CDH2), snail family transcriptional repressor 2 (SNAI2), and twist family bHLH transcription factor 1 (TWIST1).
Conclusions: PPE inhibited cell growth, induced G2/M arrest, and suppressed the migration and invasion of J82 cells. BC tumor growth in vivo was also inhibited by PPE. Our results lay the foundation for further studies on the antitumor mechanisms of PPE.
Keywords: Urinary bladder neoplasm; cell cycle; epithelial-mesenchymal transition (EMT); medicine, Chinese traditional.
2020 Translational Cancer Research. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr-20-1512). The authors have no conflicts of interest to declare.
Figures

Similar articles
-
Polyphyllin II inhibits human bladder cancer migration and invasion by regulating EMT-associated factors and MMPs.Oncol Lett. 2020 Sep;20(3):2928-2936. doi: 10.3892/ol.2020.11839. Epub 2020 Jul 9. Oncol Lett. 2020. PMID: 32782609 Free PMC article.
-
Paris polyphylla ethanol extract and polyphyllin I ameliorate adenomyosis by inhibiting epithelial-mesenchymal transition.Phytomedicine. 2024 May;127:155461. doi: 10.1016/j.phymed.2024.155461. Epub 2024 Feb 22. Phytomedicine. 2024. PMID: 38452697
-
Paris polyphylla Suppresses Proliferation and Vasculogenic Mimicry of Human Osteosarcoma Cells and Inhibits Tumor Growth In Vivo.Am J Chin Med. 2017;45(3):575-598. doi: 10.1142/S0192415X17500343. Epub 2017 Apr 6. Am J Chin Med. 2017. PMID: 28385078
-
Root extract of Hemsleya amabilis Diels suppresses renal cell carcinoma cell growth through inducing apoptosis and G2/M phase arrest via PI3K/AKT signaling pathway.J Ethnopharmacol. 2024 Jan 10;318(Pt B):117014. doi: 10.1016/j.jep.2023.117014. Epub 2023 Aug 7. J Ethnopharmacol. 2024. PMID: 37557938
-
Paris Polyphylla-Derived Saponins Inhibit Growth of Bladder Cancer Cells by Inducing Mutant P53 Degradation While Up-Regulating CDKN1A Expression.Curr Urol. 2018 Mar;11(3):131-138. doi: 10.1159/000447207. Epub 2018 Feb 20. Curr Urol. 2018. PMID: 29692692 Free PMC article.
Cited by
-
Polyphyllin Ⅲ regulates EMT of lung cancer cells through GSK-3β/β-catenin pathway.Ann Med Surg (Lond). 2024 Jan 15;86(3):1376-1385. doi: 10.1097/MS9.0000000000001629. eCollection 2024 Mar. Ann Med Surg (Lond). 2024. PMID: 38463106 Free PMC article.
-
Paris polyphylla Sm. Induces Reactive Oxygen Species and Caspase 3-Mediated Apoptosis in Colorectal Cancer Cells In Vitro and Potentiates the Therapeutic Significance of Fluorouracil and Cisplatin.Plants (Basel). 2023 Mar 25;12(7):1446. doi: 10.3390/plants12071446. Plants (Basel). 2023. PMID: 37050072 Free PMC article.
-
Polyphyllin II inhibits breast cancer cell proliferation via the PI3K/Akt signaling pathway.Mol Med Rep. 2024 Dec;30(6):224. doi: 10.3892/mmr.2024.13348. Epub 2024 Oct 4. Mol Med Rep. 2024. PMID: 39364737 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
