Pathophysiological Consequences of At-Risk Alcohol Use; Implications for Comorbidity Risk in Persons Living With Human Immunodeficiency Virus
- PMID: 35115952
- PMCID: PMC8804300
- DOI: 10.3389/fphys.2021.758230
Pathophysiological Consequences of At-Risk Alcohol Use; Implications for Comorbidity Risk in Persons Living With Human Immunodeficiency Virus
Abstract
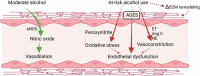
At-risk alcohol use is a significant risk factor associated with multisystemic pathophysiological effects leading to multiorgan injury and contributing to 5.3% of all deaths worldwide. The alcohol-mediated cellular and molecular alterations are particularly salient in vulnerable populations, such as people living with HIV (PLWH), diminishing their physiological reserve, and accelerating the aging process. This review presents salient alcohol-associated mechanisms involved in exacerbation of cardiometabolic and neuropathological comorbidities and their implications in the context of HIV disease. The review integrates consideration of environmental factors, such as consumption of a Western diet and its interactions with alcohol-induced metabolic and neurocognitive dyshomeostasis. Major alcohol-mediated mechanisms that contribute to cardiometabolic comorbidity include impaired substrate utilization and storage, endothelial dysfunction, dysregulation of the renin-angiotensin-aldosterone system, and hypertension. Neuroinflammation and loss of neurotrophic support in vulnerable brain regions significantly contribute to alcohol-associated development of neurological deficits and alcohol use disorder risk. Collectively, evidence suggests that at-risk alcohol use exacerbates cardiometabolic and neurocognitive pathologies and accelerates biological aging leading to the development of geriatric comorbidities manifested as frailty in PLWH.
Keywords: HIV; alcohol; cardiometabolic comorbidity; diet; neuropathology.
Copyright © 2022 Simon, Edwards and Molina.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Impact of Alcohol on HIV Disease Pathogenesis, Comorbidities and Aging: Integrating Preclinical and Clinical Findings.Alcohol Alcohol. 2018 Jul 1;53(4):439-447. doi: 10.1093/alcalc/agy016. Alcohol Alcohol. 2018. PMID: 29546271 Free PMC article. Review.
-
Geriatric Syndromes in People Living with HIV Associated with Ageing and Increasing Comorbidities: Implications for Neurocognitive Complications of HIV Infection.Curr Top Behav Neurosci. 2021;50:301-327. doi: 10.1007/7854_2019_119. Curr Top Behav Neurosci. 2021. PMID: 31907879
-
Neurocognitive SuperAging in Older Adults Living With HIV: Demographic, Neuromedical and Everyday Functioning Correlates.J Int Neuropsychol Soc. 2019 May;25(5):507-519. doi: 10.1017/S1355617719000018. Epub 2019 Mar 20. J Int Neuropsychol Soc. 2019. PMID: 30890191 Free PMC article.
-
Psychosocial stressors and alcohol use, severity, and treatment receipt across human immunodeficiency virus (HIV) status in a nationally representative sample of US residents.Subst Abus. 2017 Jul-Sep;38(3):269-277. doi: 10.1080/08897077.2016.1268238. Epub 2016 Dec 7. Subst Abus. 2017. PMID: 27925867 Free PMC article.
-
Frailty in People Living with HIV.Curr HIV/AIDS Rep. 2020 Jun;17(3):226-236. doi: 10.1007/s11904-020-00494-2. Curr HIV/AIDS Rep. 2020. PMID: 32394155 Review.
Cited by
-
Differential Regulation of Tachykinin and Opioid System Gene Expression in Brain and Immune Cells of Chronic Binge Alcohol-Treated Simian Immunodeficiency Virus-Infected Macaques.AIDS Res Hum Retroviruses. 2023 May;39(5):232-240. doi: 10.1089/AID.2022.0122. Epub 2023 Mar 10. AIDS Res Hum Retroviruses. 2023. PMID: 36762939 Free PMC article.
-
The hidden risks of alcohol use for pain relief.Alcohol Clin Exp Res (Hoboken). 2023 Feb;47(2):209-210. doi: 10.1111/acer.15005. Epub 2023 Jan 3. Alcohol Clin Exp Res (Hoboken). 2023. PMID: 36575055 Free PMC article. No abstract available.
-
Cellular Bioenergetics: Experimental Evidence for Alcohol-induced Adaptations.Function (Oxf). 2022 Aug 24;3(5):zqac039. doi: 10.1093/function/zqac039. eCollection 2022. Function (Oxf). 2022. PMID: 36120487 Free PMC article. Review.
References
-
- Achhra A. C., Sabin C., Ryom L., Hatleberg C., Antonella D’aminio M., De Wit S., et al. (2018). Body mass index and the risk of serious Non-AIDS events and all-cause mortality in treated HIV-Positive individuals: D: a: D cohort analysis. J. Acquir. Immune Defic. Syndr. 78 579–588. 10.1097/QAI.0000000000001722 - DOI - PubMed
-
- Adams R. S., Campbell-Sills L., Stein M. B., Sun X., Larson M. J., Kessler R. C., et al. (2020). The association of lifetime and deployment-acquired traumatic brain injury with postdeployment binge and heavy drinking. J. Head Trauma Rehabil. 35 27–36. 10.1097/HTR.0000000000000508 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

