cGAS exacerbates Schistosoma japonicum infection in a STING-type I IFN-dependent and independent manner
- PMID: 35108342
- PMCID: PMC8809611
- DOI: 10.1371/journal.ppat.1010233
cGAS exacerbates Schistosoma japonicum infection in a STING-type I IFN-dependent and independent manner
Abstract
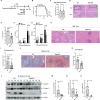
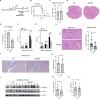
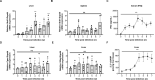
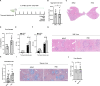
Schistosomiasis, which is caused by infection with Schistosoma spp., is characterized by granuloma and fibrosis in response to egg deposition. Pattern recognition receptors are important to sense invading Schistosoma, triggering an innate immune response, and subsequently shaping adaptive immunity. Cyclic GMP-AMP synthase (cGAS) was identified as a major cytosolic DNA sensor, which catalyzes the formation of cyclic GMP-AMP (cGAMP), a critical second messenger for the activation of the adaptor protein stimulator of interferon genes (STING). The engagement of STING by cGAMP leads to the activation of TANK-binding kinase 1 (TBK1), interferon regulatory factor 3 (IRF3), and the subsequent type I interferon (IFN) response. cGAS is suggested to regulate infectious diseases, autoimmune diseases, and cancer. However, the function of cGAS in helminth infection is unclear. In this study, we found that Cgas deficiency enhanced the survival of mice infected with S. japonicum markedly, without affecting the egg load in the liver. Consistently, Cgas deletion alleviated liver pathological impairment, reduced egg granuloma formation, and decreased fibrosis severity. In contrast, Sting deletion reduced the formation of egg granulomas markedly, but not liver fibrosis. Notably, Cgas or Sting deficiency reduced the production of IFNβ drastically in mice infected with S. japonicum. Intriguingly, intravenous administration of recombinant IFNβ exacerbated liver damage and promoted egg granuloma formation, without affecting liver fibrosis. Clodronate liposome-mediated depletion of macrophages indicated that macrophages are the major type of cells contributing to the induction of the type I IFN response during schistosome infection. Moreover, cGAS is important for type I IFN production and phosphorylation of TBK1 and IRF3 in response to stimulation with S. japonicum egg- or adult worm-derived DNA in macrophages. Our results clarified the immunomodulatory effect of cGAS in the regulation of liver granuloma formation during S. japonicum infection, involving sensing schistosome-derived DNA and producing type I IFN. Additionally, we showed that cGAS regulates liver fibrosis in a STING-type I-IFN-independent manner.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
African Swine Fever Virus Armenia/07 Virulent Strain Controls Interferon Beta Production through the cGAS-STING Pathway.J Virol. 2019 May 29;93(12):e02298-18. doi: 10.1128/JVI.02298-18. Print 2019 Jun 15. J Virol. 2019. PMID: 30918080 Free PMC article.
-
The cGas-Sting Signaling Pathway Is Required for the Innate Immune Response Against Ectromelia Virus.Front Immunol. 2018 Jun 14;9:1297. doi: 10.3389/fimmu.2018.01297. eCollection 2018. Front Immunol. 2018. PMID: 29963044 Free PMC article.
-
Salmonella Induces the cGAS-STING-Dependent Type I Interferon Response in Murine Macrophages by Triggering mtDNA Release.mBio. 2022 Jun 28;13(3):e0363221. doi: 10.1128/mbio.03632-21. Epub 2022 May 23. mBio. 2022. PMID: 35604097 Free PMC article.
-
The mechanism of double-stranded DNA sensing through the cGAS-STING pathway.Cytokine Growth Factor Rev. 2014 Dec;25(6):641-8. doi: 10.1016/j.cytogfr.2014.06.006. Epub 2014 Jun 22. Cytokine Growth Factor Rev. 2014. PMID: 25007740 Free PMC article. Review.
-
DNA sensor cGAS-mediated immune recognition.Protein Cell. 2016 Nov;7(11):777-791. doi: 10.1007/s13238-016-0320-3. Epub 2016 Sep 30. Protein Cell. 2016. PMID: 27696330 Free PMC article. Review.
Cited by
-
The multifaceted functions of cGAS.J Mol Cell Biol. 2022 Sep 15;14(5):mjac031. doi: 10.1093/jmcb/mjac031. J Mol Cell Biol. 2022. PMID: 35536585 Free PMC article. Review.
-
Leishmania kinetoplast DNA contributes to parasite burden in infected macrophages: Critical role of the cGAS-STING-TBK1 signaling pathway in macrophage parasitemia.Front Immunol. 2022 Nov 2;13:1007070. doi: 10.3389/fimmu.2022.1007070. eCollection 2022. Front Immunol. 2022. PMID: 36405710 Free PMC article.
-
The battle between the innate immune cGAS-STING signaling pathway and human herpesvirus infection.Front Immunol. 2023 Aug 2;14:1235590. doi: 10.3389/fimmu.2023.1235590. eCollection 2023. Front Immunol. 2023. PMID: 37600809 Free PMC article. Review.
-
Pattern recognition receptor signaling and innate immune responses to schistosome infection.Front Cell Infect Microbiol. 2022 Oct 21;12:1040270. doi: 10.3389/fcimb.2022.1040270. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36339337 Free PMC article. Review.
-
The dual function of cGAS-STING signaling axis in liver diseases.Acta Pharmacol Sin. 2024 Jun;45(6):1115-1129. doi: 10.1038/s41401-023-01220-5. Epub 2024 Jan 17. Acta Pharmacol Sin. 2024. PMID: 38233527 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

