The Putative Drosophila TMEM184B Ortholog Tmep Ensures Proper Locomotion by Restraining Ectopic Firing at the Neuromuscular Junction
- PMID: 35107803
- PMCID: PMC9018515
- DOI: 10.1007/s12035-022-02760-3
The Putative Drosophila TMEM184B Ortholog Tmep Ensures Proper Locomotion by Restraining Ectopic Firing at the Neuromuscular Junction
Abstract
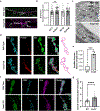
TMEM184B is a putative seven-pass membrane protein that promotes axon degeneration after injury. TMEM184B mutation causes aberrant neuromuscular architecture and sensory and motor behavioral defects in mice. The mechanism through which TMEM184B causes neuromuscular defects is unknown. We employed Drosophila melanogaster to investigate the function of the closely related gene, Tmep (CG12004), at the neuromuscular junction. We show that Tmep is required for full adult viability and efficient larval locomotion. Tmep mutant larvae have a reduced body contraction rate compared to controls, with stronger deficits in females. In recordings from body wall muscles, Tmep mutants show substantial hyperexcitability, with many postsynaptic potentials fired in response to a single stimulation, consistent with a role for Tmep in restraining synaptic excitability. Additional branches and satellite boutons at Tmep mutant neuromuscular junctions are consistent with an activity-dependent synaptic overgrowth. Tmep is expressed in endosomes and synaptic vesicles within motor neurons, suggesting a possible role in synaptic membrane trafficking. Using RNAi knockdown, we show that Tmep is required in motor neurons for proper larval locomotion and excitability, and that its reduction increases levels of presynaptic calcium. Locomotor defects can be rescued by presynaptic knockdown of endoplasmic reticulum calcium channels or by reducing evoked release probability, further suggesting that excess synaptic activity drives behavioral deficiencies. Our work establishes a critical function for Tmep in the regulation of synaptic transmission and locomotor behavior.
Keywords: Calcium; Epilepsy; Excitability; Neuromuscular junction; Synapse; TMEM184B.
© 2022. The Author(s), under exclusive licence to Springer Science+Business Media, LLC, part of Springer Nature.
Conflict of interest statement
Conflicts of interest/Competing interests
The authors declare no competing financial interests.
Figures



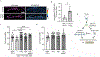
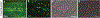
Similar articles
-
Drosophila-Cdh1 (Rap/Fzr) a regulatory subunit of APC/C is required for synaptic morphology, synaptic transmission and locomotion.Int J Dev Neurosci. 2013 Nov;31(7):624-33. doi: 10.1016/j.ijdevneu.2013.07.002. Epub 2013 Aug 7. Int J Dev Neurosci. 2013. PMID: 23933137 Free PMC article.
-
The Drosophila metabotropic glutamate receptor DmGluRA regulates activity-dependent synaptic facilitation and fine synaptic morphology.J Neurosci. 2004 Oct 13;24(41):9105-16. doi: 10.1523/JNEUROSCI.2724-04.2004. J Neurosci. 2004. PMID: 15483129 Free PMC article.
-
A TRPV channel in Drosophila motor neurons regulates presynaptic resting Ca2+ levels, synapse growth, and synaptic transmission.Neuron. 2014 Nov 19;84(4):764-77. doi: 10.1016/j.neuron.2014.09.030. Epub 2014 Oct 30. Neuron. 2014. PMID: 25451193 Free PMC article.
-
Synaptic homeostasis on the fast track.Neuron. 2006 Nov 22;52(4):569-71. doi: 10.1016/j.neuron.2006.11.006. Neuron. 2006. PMID: 17114040 Review.
-
Electrophysiological analysis of synaptic transmission in Drosophila.Wiley Interdiscip Rev Dev Biol. 2017 Sep;6(5):10.1002/wdev.277. doi: 10.1002/wdev.277. Epub 2017 May 24. Wiley Interdiscip Rev Dev Biol. 2017. PMID: 28544556 Free PMC article. Review.
Cited by
-
Pathogenic variants in TMEM184B cause a neurodevelopmental syndrome via alteration of metabolic signaling.medRxiv [Preprint]. 2024 Jul 1:2024.06.27.24309417. doi: 10.1101/2024.06.27.24309417. medRxiv. 2024. PMID: 39006436 Free PMC article. Preprint.
-
Transmembrane protein 184B (TMEM184B) promotes expression of synaptic gene networks in the mouse hippocampus.BMC Genomics. 2023 Sep 20;24(1):559. doi: 10.1186/s12864-023-09676-9. BMC Genomics. 2023. PMID: 37730546 Free PMC article.
References
-
- Schneggenburger R, Neher E (2005) Presynaptic calcium and control of vesicle fusion. Curr. Opin. Neurobiol 15:266–274 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

