H84T BanLec has broad spectrum antiviral activity against human herpesviruses in cells, skin, and mice
- PMID: 35102178
- PMCID: PMC8803833
- DOI: 10.1038/s41598-022-05580-6
H84T BanLec has broad spectrum antiviral activity against human herpesviruses in cells, skin, and mice
Abstract
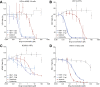
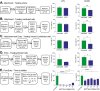
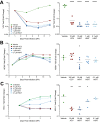
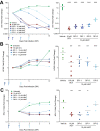
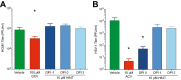
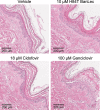
H84T BanLec is a molecularly engineered lectin cloned from bananas with broad-spectrum antiviral activity against several RNA viruses. H84T BanLec dimers bind glycoproteins containing high-mannose N-glycans on the virion envelope, blocking attachment, entry, uncoating, and spread. It was unknown whether H84T BanLec is effective against human herpesviruses varicella-zoster virus (VZV), human cytomegalovirus (HCMV), and herpes simplex virus 1 (HSV-1), which express high-mannose N-linked glycoproteins on their envelopes. We evaluated H84T BanLec against VZV-ORF57-Luc, TB40/E HCMV-fLuc-eGFP, and HSV-1 R8411 in cells, skin organ culture, and mice. The H84T BanLec EC50 was 0.025 µM for VZV (SI50 = 4000) in human foreskin fibroblasts (HFFs), 0.23 µM for HCMV (SI50 = 441) in HFFs, and 0.33 µM for HSV-1 (SI50 = 308) in Vero cells. Human skin was obtained from reduction mammoplasties and prepared for culture. Skin was infected and cultured up to 14 days. H84T BanLec prevented VZV, HCMV and HSV-1 spread in skin at 10 µM in the culture medium, and also exhibited dose-dependent antiviral effects. Additionally, H84T BanLec arrested virus spread when treatment was delayed. Histopathology of HCMV-infected skin showed no overt toxicity when H84T BanLec was present in the media. In athymic nude mice with human skin xenografts (NuSkin mice), H84T BanLec reduced VZV spread when administered subcutaneously prior to intraxenograft virus inoculation. This is the first demonstration of H84T BanLec effectiveness against DNA viruses. H84T BanLec may have additional unexplored activity against other, clinically relevant, glycosylated viruses.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
A Novel Human Skin Tissue Model To Study Varicella-Zoster Virus and Human Cytomegalovirus.J Virol. 2020 Oct 27;94(22):e01082-20. doi: 10.1128/JVI.01082-20. Print 2020 Oct 27. J Virol. 2020. PMID: 32878893 Free PMC article.
-
Inhibition of Ebola Virus by a Molecularly Engineered Banana Lectin.PLoS Negl Trop Dis. 2019 Jul 29;13(7):e0007595. doi: 10.1371/journal.pntd.0007595. eCollection 2019 Jul. PLoS Negl Trop Dis. 2019. PMID: 31356611 Free PMC article.
-
A molecularly engineered, broad-spectrum anti-coronavirus lectin inhibits SARS-CoV-2 and MERS-CoV infection in vivo.Cell Rep Med. 2022 Oct 18;3(10):100774. doi: 10.1016/j.xcrm.2022.100774. Epub 2022 Sep 29. Cell Rep Med. 2022. PMID: 36195094 Free PMC article.
-
Herpesviridae and novel inhibitors.Antivir Ther. 2009;14(8):1051-64. doi: 10.3851/IMP1467. Antivir Ther. 2009. PMID: 20032535 Review.
-
The antiviral spectrum of (E)-5-(2-bromovinyl)-2'-deoxyuridine.J Antimicrob Chemother. 1984 Aug;14 Suppl A:85-95. doi: 10.1093/jac/14.suppl_a.85. J Antimicrob Chemother. 1984. PMID: 6092320 Review.
Cited by
-
Targeting glycans for CAR therapy: The advent of sweet CARs.Mol Ther. 2022 Sep 7;30(9):2881-2890. doi: 10.1016/j.ymthe.2022.07.006. Epub 2022 Jul 12. Mol Ther. 2022. PMID: 35821636 Free PMC article. Review.
-
Applications of mannose-binding lectins and mannan glycoconjugates in nanomedicine.J Nanopart Res. 2022;24(11):228. doi: 10.1007/s11051-022-05594-1. Epub 2022 Nov 4. J Nanopart Res. 2022. PMID: 36373057 Free PMC article. Review.
-
Prodrug Strategies for the Development of β-l-5-((E)-2-Bromovinyl)-1-((2S,4S)-2-(hydroxymethyl)-1,3-(dioxolane-4-yl))uracil (l-BHDU) against Varicella Zoster Virus (VZV).J Med Chem. 2023 May 25;66(10):7038-7053. doi: 10.1021/acs.jmedchem.3c00545. Epub 2023 May 4. J Med Chem. 2023. PMID: 37140467 Free PMC article.
-
Development of Robust Varicella Zoster Virus Luciferase Reporter Viruses for In Vivo Monitoring of Virus Growth and Its Antiviral Inhibition in Culture, Skin, and Humanized Mice.Viruses. 2022 Apr 15;14(4):826. doi: 10.3390/v14040826. Viruses. 2022. PMID: 35458556 Free PMC article.
-
Humanized Severe Combined Immunodeficient (SCID) Mouse Models for Varicella-Zoster Virus Pathogenesis.Curr Top Microbiol Immunol. 2023;438:135-161. doi: 10.1007/82_2022_255. Curr Top Microbiol Immunol. 2023. PMID: 35292858
References
-
- Payne S. Chapter 34: Family Herpesviridae. Elsevier Science; 2017.
-
- McQuillan, G., Kruszon-Moran, D., Flagg, E. W., Paulose-Ram, R. Prevalence of herpes simplex virus type 1 and type 2 in persons aged 14–49: United States, 2015–2016. NCHS Data Brief 1–8 (2018). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

