MicroRNA-92a-3p Enhances Cisplatin Resistance by Regulating Krüppel-Like Factor 4-Mediated Cell Apoptosis and Epithelial-to-Mesenchymal Transition in Cervical Cancer
- PMID: 35095494
- PMCID: PMC8795743
- DOI: 10.3389/fphar.2021.783213
MicroRNA-92a-3p Enhances Cisplatin Resistance by Regulating Krüppel-Like Factor 4-Mediated Cell Apoptosis and Epithelial-to-Mesenchymal Transition in Cervical Cancer
Abstract
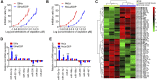
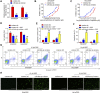
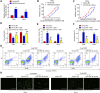
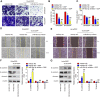
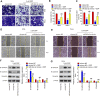
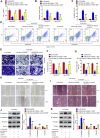
Recent studies have confirmed the existence and key roles of microRNA (miRNAs) in cancer drug resistance, including cervical cancer (CC). The present study aims to establish a novel role for miR-92a-3p and its associated gene networks in cisplatin (DDP) resistance of CC. First, the disparities in miRNA expression between CC tissues and adjacent normal tissues were screened based on GSE19611 microarray data that retrieved from Gene Expression Omnibus (GEO), and we identified several miRs that were significantly downregulated or upregulated in CC tissues including miR-92a-3p. Moreover, miR-92a-3p was significantly up-regulated in DDP-resistant cells and was the most differently expressed miRNA. Functionally, knockdown of miR-92a-3p increased the sensitivity of DDP-resistant cells to DDP via inhibiting cell proliferation, migration and invasion, and promoting apoptosis. Conversely, overexpression of miR-92a-3p significantly induced DDP resistance in CC parental cells including HeLa and SiHa cells. Moreover, Krüppel-like factor 4 (KLF4) was identified as a direct target of miR-92a-3p, and an obvious inverse correlation was observed between the expression of miR-92a-3p and KLF4 in 40 pairs of cancer tissues. Furthermore, KLF4 knockdown reversed the promoting effect of miR-92a-3p inhibition on DDP sensitivity in DDP-resistant CC cells. Besides, high expression of miR-92a-3p was associated with DDP resistance, as well as a short overall survival in clinic. Taken together, these findings provide important evidence that miR-92a-3p targets KLF4 and is significant in DDP resistance in CC, indicating that miR-92a-3p may be an attractive target to increase DDP sensitivity in clinical CC treatment.
Keywords: KLF4; apoptosis; cervical cancer; cisplatin resistance; miR-92a-3p.
Copyright © 2022 Yang, Hai, Dong, Zhang and Duan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
miR-34b-3p-mediated regulation of STC2 and FN1 enhances chemosensitivity and inhibits proliferation in cervical cancer.Acta Biochim Biophys Sin (Shanghai). 2024 May 25;56(5):740-752. doi: 10.3724/abbs.2024009. Acta Biochim Biophys Sin (Shanghai). 2024. PMID: 38477044 Free PMC article.
-
MicroRNA-125a-5p targets LIM kinase 1 to inhibit cisplatin resistance of cervical cancer cells.Oncol Lett. 2021 May;21(5):392. doi: 10.3892/ol.2021.12653. Epub 2021 Mar 18. Oncol Lett. 2021. PMID: 33777215 Free PMC article.
-
MicroRNA-574-3p regulates epithelial mesenchymal transition and cisplatin resistance via targeting ZEB1 in human gastric carcinoma cells.Gene. 2019 Jun 5;700:110-119. doi: 10.1016/j.gene.2019.03.043. Epub 2019 Mar 24. Gene. 2019. PMID: 30917930
-
LINC01915 Facilitates the Conversion of Normal Fibroblasts into Cancer-Associated Fibroblasts Induced by Colorectal Cancer-Derived Extracellular Vesicles through the miR-92a-3p/KLF4/CH25H Axis.ACS Biomater Sci Eng. 2021 Nov 8;7(11):5255-5268. doi: 10.1021/acsbiomaterials.1c00611. Epub 2021 Oct 13. ACS Biomater Sci Eng. 2021. PMID: 34643375
-
Current research advances in microRNA-mediated regulation of Krüppel-like factor 4 in cancer: a narrative review.Ann Transl Med. 2021 Jun;9(11):948. doi: 10.21037/atm-21-2347. Ann Transl Med. 2021. PMID: 34350263 Free PMC article. Review.
Cited by
-
miR-92a-3p promotes breast cancer proliferation by regulating the KLF2/BIRC5 axis.Thorac Cancer. 2022 Nov;13(21):2992-3000. doi: 10.1111/1759-7714.14648. Epub 2022 Sep 13. Thorac Cancer. 2022. PMID: 36100919 Free PMC article.
-
Non-coding RNA in cancer drug resistance: Underlying mechanisms and clinical applications.Front Oncol. 2022 Aug 17;12:951864. doi: 10.3389/fonc.2022.951864. eCollection 2022. Front Oncol. 2022. PMID: 36059609 Free PMC article. Review.
-
miR-92a-3p regulates cisplatin-induced cancer cell death.Cell Death Dis. 2023 Sep 13;14(9):603. doi: 10.1038/s41419-023-06125-z. Cell Death Dis. 2023. PMID: 37704611 Free PMC article.
-
Natural products reverse cancer multidrug resistance.Front Pharmacol. 2024 Mar 8;15:1348076. doi: 10.3389/fphar.2024.1348076. eCollection 2024. Front Pharmacol. 2024. PMID: 38572428 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

